History of the Atom (Atomic Theory)
Summary
TLDRThis script explores the historical development of atomic theory, from Democritus' concept of indivisible particles to modern quantum mechanics. It discusses Democritus' idea of atoms as the smallest, uncuttable units, Aristotle's opposing elemental theory, and John Dalton's atomic theory with its five postulates. The script then delves into J.J. Thomson's discovery of electrons, Rutherford's identification of the atomic nucleus with protons, and Niels Bohr's model of electrons in discrete energy levels. It concludes with de Broglie's wave-particle duality, Heisenberg's uncertainty principle, Schrödinger's electron cloud model, and Chadwick's discovery of the neutron, painting a comprehensive picture of atomic structure.
Takeaways
- 😀 Democritus, an ancient Greek philosopher, was the first to propose the concept of atoms, which he described as indivisible and indestructible particles.
- 🔬 Aristotle, a contemporary of Democritus, disagreed with the atomic theory and instead believed that matter was composed of five basic elements: earth, water, air, fire, and ether.
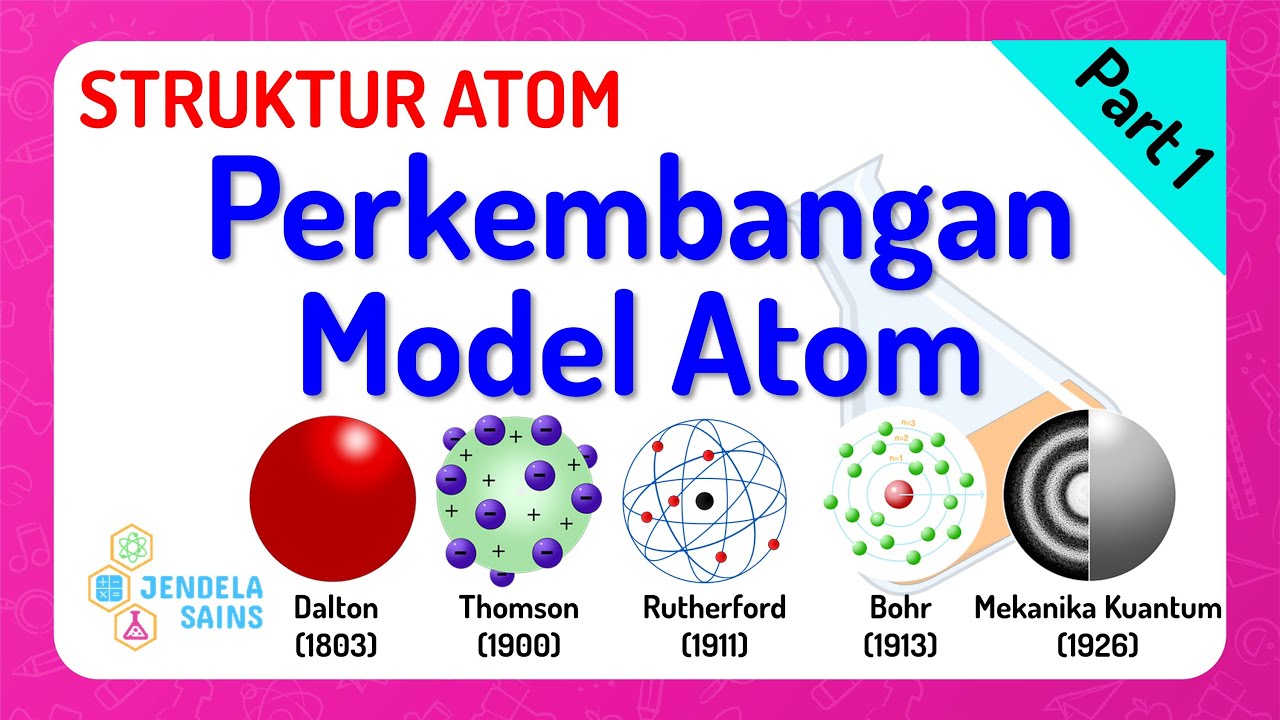
- 🧪 John Dalton's atomic theory, developed in the early 1800s, was the first to be based on experimental evidence and included postulates about the composition and behavior of atoms.
- 🌐 J.J. Thompson discovered the electron, a negatively charged particle within the atom, by studying cathode rays and their deflection in electric and magnetic fields.
- 🍪 Thompson's 'Plum Pudding Model' suggested that electrons were embedded within a positively charged 'pudding' representing the rest of the atom.
- 🔋 Ernest Rutherford's gold foil experiment led to the discovery of the proton and the understanding that atoms are mostly empty space with a dense, positively charged nucleus.
- 🌌 Niels Bohr introduced the concept of electron energy levels, proposing that electrons orbit the nucleus in discrete paths and can only exist at certain energy levels.
- 🌊 Louis de Broglie proposed that electrons behave as waves, leading to a better understanding of their energy and position through the concept of wave-particle duality.
- 📊 Werner Heisenberg's uncertainty principle states that it is impossible to know both the exact position and momentum of an electron simultaneously.
- 🌐 Erwin Schrödinger developed a wave equation that describes the probability of finding an electron in a particular region around the nucleus, known as the electron cloud model.
- 💥 James Chadwick's discovery of the neutron completed the modern understanding of the atomic nucleus, which contains both protons and neutrons.
Q & A
Who was Democritus and what was his contribution to the concept of the atom?
-Democritus was an ancient Greek philosopher who lived around 400 BC. He was the first person to come up with the idea of an atom, using the word 'atomos' which means uncuttable or smallest indivisible particle. His theory suggested that all matter could be broken down into these smallest particles.
What was Aristotle's view on the composition of matter, and how did it differ from Democritus'?
-Aristotle believed that matter was made up of five basic elements: earth, water, air, fire, and ether. This differed from Democritus' view that matter was composed of tiny, indivisible particles called atoms.
How did John Dalton's atomic theory build upon Democritus' ideas?
-John Dalton developed the atomic theory based on experimental evidence and observations. His theory included five main postulates: all matter is composed of atoms, atoms cannot be created or destroyed, all atoms of the same element are identical, chemical reactions occur when atoms are rearranged, and compounds are formed by the combination of two or more different kinds of atoms.
What was J.J. Thompson's contribution to the understanding of atomic structure?
-J.J. Thompson discovered that the atom could be divided and identified a negatively charged part of the atom called the electron. He used a cathode ray tube to visualize these particles and concluded that they were negatively charged.
Can you describe Ernest Rutherford's gold foil experiment and its implications for atomic theory?
-Ernest Rutherford fired alpha particles at a thin sheet of gold foil and observed that some particles scattered or deflected backwards. This led to the conclusion that there must be a very massive, positively charged particle at the center of the atom, which he called the nucleus, and that the atom was mostly empty space with electrons circling around the nucleus.
What is the significance of Niels Bohr's model of the atom?
-Niels Bohr's model introduced the concept that electrons travel around the atom in discrete energy levels and can only exist on these levels. He also introduced the idea of a quantum of energy, which is the exact amount of energy needed for an electron to move from one energy level to another.
What is the Heisenberg uncertainty principle, and how does it relate to electrons in an atom?
-The Heisenberg uncertainty principle, proposed by Werner Heisenberg, states that it is impossible to know both the exact position and momentum of a particle, such as an electron, at the same time. This principle implies that electrons in an atom cannot be precisely located in specific rings but rather exist in a cloud of probability.
How did Louis de Broglie's work contribute to the understanding of electron behavior?
-Louis de Broglie proposed that electrons could be described as waves rather than particles, suggesting that their energy could be better understood by looking at their wavelength. This concept laid the groundwork for the electron cloud model of the atom.
What is the electron cloud model, and how does it differ from the earlier models of the atom?
-The electron cloud model, developed in part by Erwin Schrödinger, suggests that electrons do not exist in fixed orbits but rather in regions of probability around the nucleus. This model differs from earlier models, like the plum pudding model and Bohr's model, which suggested electrons moved in fixed, circular orbits.
Who discovered the neutron, and how did this discovery impact the understanding of atomic structure?
-James Chadwick discovered the neutron, which is located in the nucleus of the atom along with protons. This discovery completed the modern understanding of the atomic nucleus as being composed of both positively charged protons and neutral neutrons, with electrons orbiting around it.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
Struktur Atom • Part 1: Perkembangan Model Atom

Kimia X - Struktur Atom #3 | Perkembangan Teori dan Model Atom

PERKEMBANGAN TEORI ATOM LENGKAP (KELAS 10)

Materi, Atom dan Sejarah Perkembangan Atom

Development of Atomic Theory: An Introduction

SCIENCE 8: Q2_WREK 1- DAY 1: GREEK PHILOSOPHERS AND THE ATOMOS ||MATATAG CURRICULUM
5.0 / 5 (0 votes)
