PERKEMBANGAN TEORI ATOM LENGKAP (KELAS 10)
Summary
TLDRThis script explores the concept of atoms, beginning with the ancient Greek philosophers' musings on the indivisible smallest unit of matter. It traces the development of atomic theory from Democritus and Aristotle to Dalton's postulates and the discovery of the electron by Thomson. The script delves into the Rutherford model, Bohr's quantum theory, and the evolution to quantum mechanics, highlighting the contributions of scientists like Schrödinger and Heisenberg. It encapsulates the journey from the philosophical idea of atoms to the complex quantum model that describes the subatomic world.
Takeaways
- 🤔 The concept of atoms was first introduced by the Greek philosopher Democritus in the 5th century BCE, suggesting that all matter is composed of indivisible particles.
- 🔬 Aristotle later proposed a different concept of atoms, suggesting that all matter is made up of four elements: fire, water, earth, and air, which can be infinitely divided.
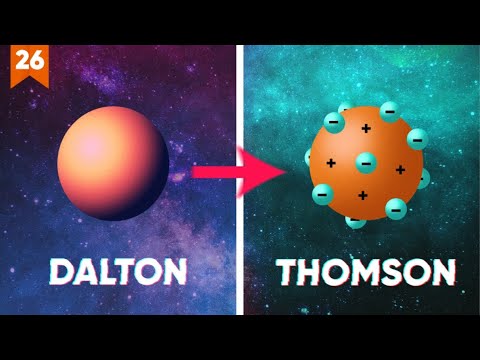
- 🧪 John Dalton, an English scientist, formulated the first modern atomic theory in the early 19th century, postulating that matter is made of small, indivisible particles called atoms.
- 🚀 Dalton's atomic theory included four postulates: atoms of the same element are identical, atoms of different elements differ, atoms combine in simple ratios to form compounds, and atoms are indestructible in chemical reactions.
- 🌐 J.J. Thomson discovered the electron in 1897 and proposed the 'plum pudding' model of the atom, suggesting that atoms consist of a positively charged sphere with electrons embedded within.
- 💥 Ernest Rutherford's gold foil experiment in 1909 revealed that atoms have a dense, positively charged nucleus with electrons orbiting around it, leading to the 'Rutherford model' of the atom.
- ⚛️ The Rutherford model had limitations, such as not explaining why electrons don't fall into the nucleus and not accounting for the spectral lines of hydrogen.
- 🌌 Niels Bohr improved the Rutherford model in 1913 by introducing quantum theory, suggesting that electrons orbit the nucleus in specific energy levels and that the atom's energy is quantized.
- 🔍 Bohr's model addressed the spectral lines of hydrogen but had its own limitations, such as not explaining the precise location and motion of electrons.
- 🌟 The development of quantum mechanics in the 1920s, with contributions from scientists like Louis de Broglie, Erwin Schrödinger, and Werner Heisenberg, introduced the concept of wave-particle duality and the uncertainty principle to atomic theory.
- 📚 The modern quantum mechanical model of the atom describes electrons as existing in 'orbitals' or regions of space with the highest probability of finding an electron, rather than fixed orbits.
Q & A
What is the concept of atoms?
-The concept of atoms is the idea that all matter is composed of small, indivisible particles known as atoms, which are the basic units of chemical elements.
Who first proposed the concept of atoms?
-The concept of atoms was first proposed by the Greek philosopher Democritus in the 5th century BCE.
What was the main flaw in Democritus' theory of atoms?
-The main flaw in Democritus' theory was that it was purely speculative and lacked empirical evidence from experiments.
What is Dalton's atomic theory?
-Dalton's atomic theory, proposed in the early 19th century, states that matter is made up of small, indivisible particles called atoms, which are identical for a given element but different from atoms of other elements.
What are the four postulates of Dalton's atomic theory?
-Dalton's four postulates are: 1) Matter is made up of atoms, 2) Atoms of the same element are identical in all respects, 3) Atoms can combine or recombine to form compounds, and 4) Atoms can form molecules with simple ratios.
What was the limitation of Dalton's theory regarding the nature of atoms?
-Dalton's theory did not explain the electrical properties of matter, the way atoms bond, or the differences between one atom and another.
Who discovered the electron and how did it impact the atomic theory?
-J.J. Thomson discovered the electron in 1897, which led to a new model of the atom that included negatively charged electrons distributed throughout the atom.
What is the Rutherford atomic model?
-The Rutherford atomic model, proposed in 1911, describes the atom as having a positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus.
What experiment did Rutherford perform to develop his atomic model?
-Rutherford performed the gold foil experiment, where alpha particles were fired at a thin gold foil, leading to the discovery of the atomic nucleus.
What is the Bohr model of the atom and how does it differ from the Rutherford model?
-The Bohr model, proposed in 1913, describes electrons as orbiting the nucleus in specific shells or energy levels. It differs from the Rutherford model by incorporating quantum theory and explaining the stability of electron orbits.
What is the quantum mechanical model of the atom and how does it address the limitations of previous models?
-The quantum mechanical model, developed in the 1920s, is based on the wave-particle duality of electrons and introduces the concept of electron orbitals. It addresses the limitations of previous models by explaining the probabilistic nature of electron positions and the atomic spectra.
What is the Heisenberg uncertainty principle and how does it relate to the quantum mechanical model of the atom?
-The Heisenberg uncertainty principle states that it is impossible to simultaneously determine the exact position and momentum of a particle. This principle is fundamental to the quantum mechanical model of the atom, as it explains why the exact position of an electron cannot be known, only the region of highest probability.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

The Atomic Universe Theory | Universe Theories Episode 4

What Is An Atom ? | Properties of Matter | Chemistry | FuseSchool

Teoría atómica (Leucipo y Demócrito) VS Teoría de los cuatro elementos (Aristóteles)

HISTORIA DEL ÁTOMO
O Modelo Atômico de Dalton x Thomson

2.2.1 - Estudo do átomo - Hipótese atômica de Leucipo e Demócrito: Teoria Atomística
5.0 / 5 (0 votes)