Development of Atomic Theory: An Introduction
Summary
TLDRThis script takes a historical journey through the understanding of atoms, from Democritus' concept of indivisible particles to Dalton's atomic theory and the discovery of the electron by J.J. Thomson. It highlights Rutherford's gold foil experiment revealing the atomic nucleus and Bohr's model of quantized electron orbits. Finally, Chadwick's identification of the neutron completes the modern atomic model, emphasizing the evolution of scientific knowledge over millennia.
Takeaways
- 📚 Ancient Greek philosopher Democritus first coined the term 'atomos', theorizing that all matter is made up of indivisible particles with unique properties.
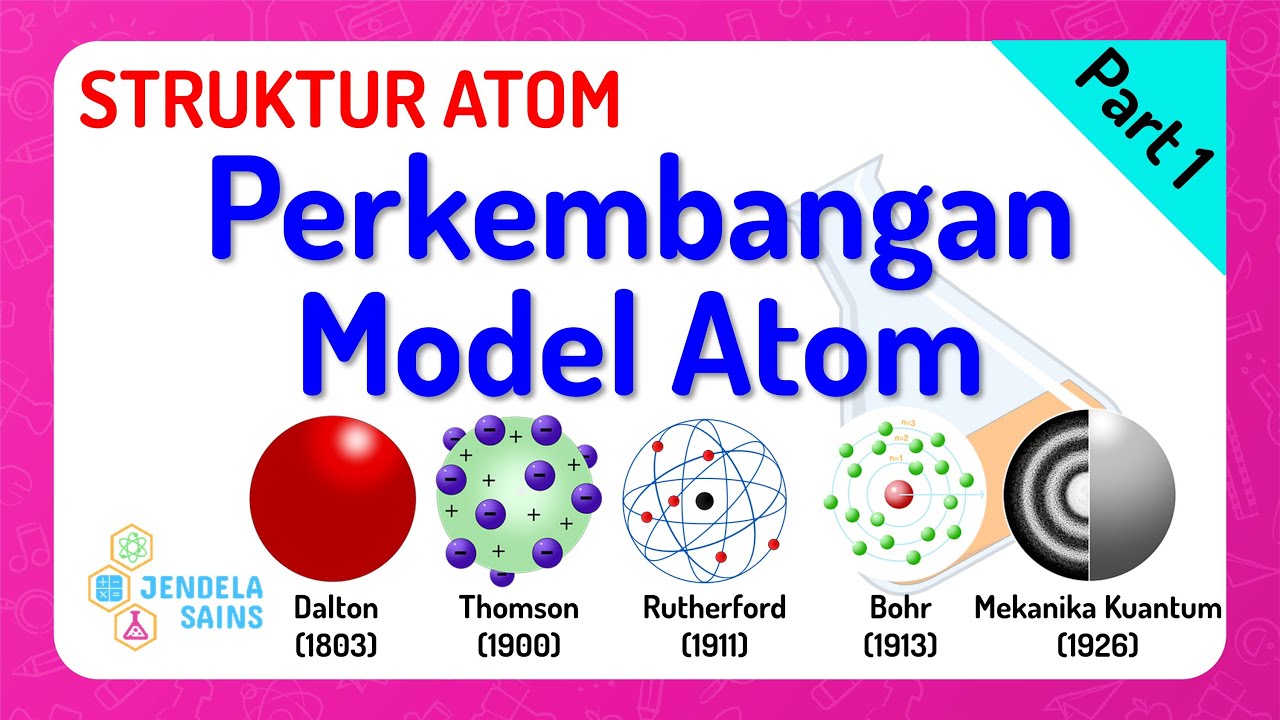
- 🔬 John Dalton advanced the atomic theory, suggesting atoms are tiny, indivisible, and combine in definite proportions, differing in kind between different elements.
- 🌐 J.J. Thomson's cathode ray experiments led to the discovery of electrons, proposing the 'plum pudding' model where electrons are scattered within a positive matrix.
- 💥 Ernest Rutherford's gold foil experiment revealed the existence of a small, dense, positively charged nucleus at the center of the atom, challenging the plum pudding model.
- 🌀 Niels Bohr introduced the concept of quantized energy levels or orbitals, where electrons occupy fixed paths around the nucleus, preventing them from spiraling inwards.
- 🧲 The nucleus contains positively charged protons, which are balanced by neutralizing particles called neutrons, as discovered by James Chadwick in the 1930s.
- 🔍 The script emphasizes the evolution of scientific understanding of the atom from purely theoretical to evidence-based through experimentation.
- 🌟 The atomic model has significantly evolved over centuries, from Democritus' initial theory to the modern understanding of atomic structure.
- 🚀 The script suggests that our current understanding of the atom is not the final word and that it will continue to evolve with future scientific advancements.
- 🌌 The journey from the early theories of the atom to the complex models we have today illustrates the dynamic nature of scientific discovery and knowledge.
- 🔬 The script highlights key scientists and their contributions to the development of atomic theory, emphasizing the collaborative and cumulative nature of scientific progress.
Q & A
Who is credited with coining the term 'atomos' and what did he theorize about the nature of matter?
-Democritus is credited with coining the term 'atomos'. He theorized that all matter was made up of tiny, indivisible particles, which retained the properties of the substances they were part of.
What was the significance of John Dalton's atomic theory and how did it differ from Democritus' early theory?
-John Dalton's atomic theory was significant because it was based on scientific observation and experimentation. Unlike Democritus, Dalton proposed that atoms are tiny, indivisible particles that combine in definite proportions, and that atoms of the same substance are identical, while atoms of different substances are different.
How did J.J. Thomson's experiments with cathode rays contribute to the understanding of atomic structure?
-J.J. Thomson's experiments with cathode rays led him to theorize the existence of tiny, negatively charged particles within the atom, which later became known as electrons. This challenged Dalton's idea of atoms being indivisible and introduced the concept of a substructure within the atom.
What is the 'raisin bun model' of the atom and who is associated with this model?
-The 'raisin bun model' of the atom, associated with J.J. Thomson, proposed that the atom consisted of a positively charged matrix with negatively charged particles, like electrons, interspersed throughout, similar to raisins in a bun.
What was the purpose of the gold foil experiment conducted by Rutherford, Geiger, and Marsden?
-The gold foil experiment was conducted to study how radioactive matter behaved, specifically to observe the transmission of positively charged alpha particles through a thin gold foil. It was expected to confirm the 'plum pudding model' of the atom.
What discovery did Rutherford, Geiger, and Marsden make during the gold foil experiment that challenged the existing atomic model?
-They discovered that some alpha particles were deflected or even reflected back when passing through the gold foil, which contradicted the idea of a neutral atom with a diffuse positive charge. This led to Rutherford's hypothesis of a small, dense, positively charged nucleus at the center of the atom.
What is the significance of Niels Bohr's model of the atom and how does it differ from previous models?
-Niels Bohr's model introduced the concept of quantized energy levels or orbitals, where electrons can only occupy specific paths around the nucleus. This model explained why electrons do not spiral into the nucleus, thus preventing the collapse of atoms.
What role did James Chadwick play in the development of atomic theory?
-James Chadwick provided evidence for the existence of neutrons, neutral particles within the atomic nucleus, which helped to explain why protons in the nucleus did not repel each other and cause the nucleus to disintegrate.
How has the understanding of atomic structure evolved from the time of Democritus to the modern era?
-The understanding of atomic structure has evolved significantly, from Democritus' concept of indivisible atoms, through Dalton's atomic theory, Thomson's discovery of electrons, Rutherford's nuclear model, Bohr's quantized energy levels, to Chadwick's discovery of neutrons, reflecting a continuous process of scientific discovery and refinement.
What is the modern interpretation of atomic structure, and how does it differ from the models mentioned in the script?
-The modern interpretation of atomic structure includes a complex model where electrons occupy probabilistic orbitals rather than fixed paths, and the nucleus contains protons and neutrons, which are made up of even smaller particles called quarks. This differs from the earlier models by incorporating quantum mechanics and the understanding of subatomic particles.
What does the script suggest about the future of our understanding of atomic structure?
-The script suggests that our understanding of atomic structure is likely to continue evolving and changing as new scientific discoveries are made, emphasizing the dynamic nature of scientific knowledge.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)