Simplest Way To Understand Boiling Point Elevation & Vapor Pressure Depression
Summary
TLDRThis educational video script explains the concepts of boiling point and vapor pressure, focusing on how they relate to colligative properties. It illustrates how water molecules vaporize at 100°C, forming water vapor with a vapor pressure equal to atmospheric pressure. The addition of particles to water increases intermolecular forces, leading to a depression in vapor pressure and an elevation in boiling point. The script introduces colligative property formulas, emphasizing factors like phantom factor and molality, which determine the extent of these effects. The goal is to simplify the understanding of vapor pressure depression and boiling point elevation for viewers.
Takeaways
- 🔍 Boiling point and vapor pressure are challenging to visualize, making them difficult to understand.
- 💧 At 100°C, the boiling point of water, the added heat breaks intermolecular forces, allowing water to vaporize.
- 🌡 The vapor pressure of water at its boiling point is equal to atmospheric pressure, enabling evaporation.
- 🌐 When particles are added to water, they create more intermolecular forces, inhibiting the vaporization of water at 100°C.
- 📉 The presence of particles depresses the vapor pressure, preventing water from vaporizing until it reaches a higher temperature.
- ⬆️ To overcome the intermolecular forces created by added particles, water must be heated to a temperature above 100°C to boil.
- 🌡️ Boiling point elevation occurs when water molecules are held in a liquid state by particles, requiring additional heat to vaporize.
- 🔄 The degree of vapor pressure depression and boiling point elevation depends on factors like the number of particles and their interaction with water molecules.
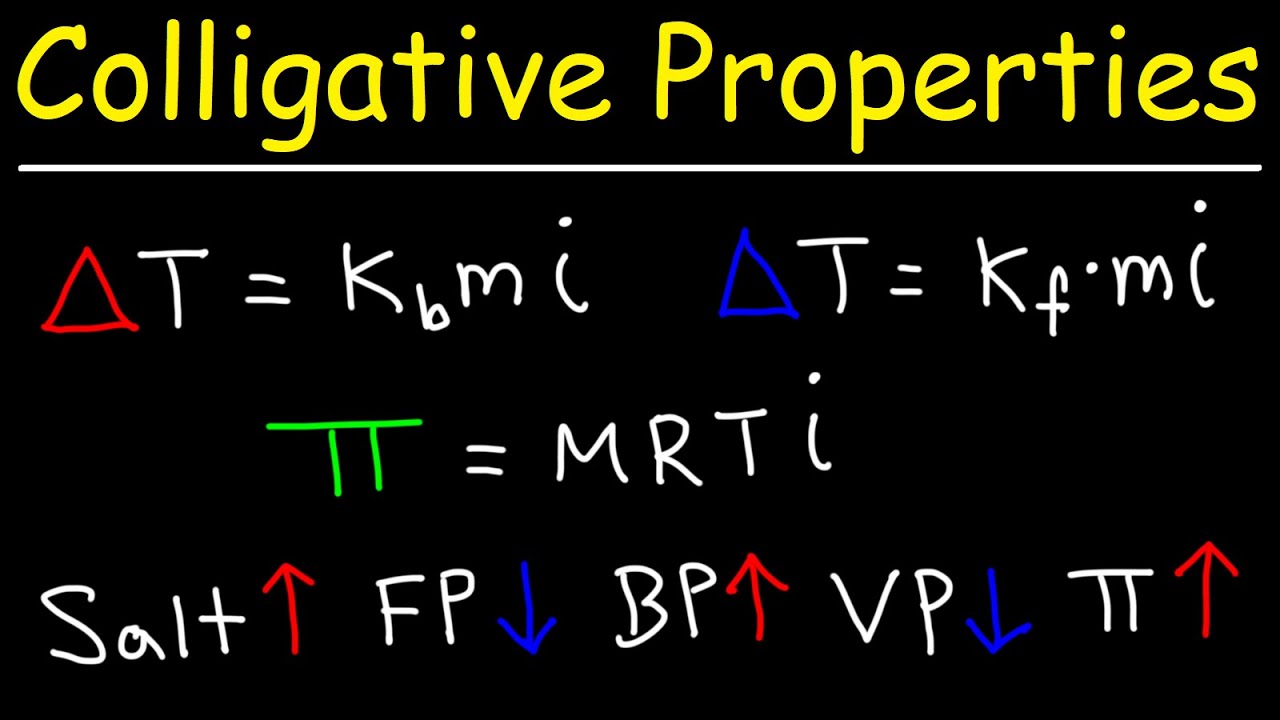
- 🔢 Colligative properties formulas, including molality and the phantom factor, help determine changes in boiling point and vapor pressure.
- 🎥 For a more detailed understanding, the video in the chemistry playlist provides a simple explanation of these concepts.
Q & A
What happens when water reaches its boiling point at 100 degrees Celsius?
-When water reaches its boiling point at 100 degrees Celsius, the heat applied goes into breaking intermolecular forces between water molecules, allowing them to vaporize into a gas, which is also known as evaporation.
What is vapor pressure and how does it relate to the boiling point of water?
-Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phases at a given temperature. It is related to the boiling point because when the vapor pressure equals the atmospheric pressure, the liquid can boil and turn into vapor.
Why is it difficult to visualize boiling point and vapor pressure?
-Boiling point and vapor pressure are difficult to visualize because they involve molecular interactions and changes at the microscopic level, which are not easily observed or understood without scientific instruments or models.
What is the role of intermolecular forces in the boiling process?
-Intermolecular forces hold water molecules together in the liquid state. During boiling, heat is used to overcome these forces, allowing water molecules to escape as vapor.
How do particles added to water affect its boiling point?
-When particles are added to water, they create additional intermolecular forces with the water molecules. This can increase the boiling point because more heat is needed to break these extra forces before the water can vaporize.
What is the term for the phenomenon where the boiling point of a solution is higher than that of pure water?
-The phenomenon where the boiling point of a solution is higher than that of pure water is called boiling point elevation.
What is vapor pressure depression and how does it differ from boiling point elevation?
-Vapor pressure depression is the reduction in vapor pressure when solutes are added to a solvent, preventing the solvent from vaporizing at its normal boiling point. Boiling point elevation, on the other hand, is the increase in the boiling point of a solution due to the presence of solutes.
What factors determine the degree of vapor pressure depression and boiling point elevation?
-The degree of vapor pressure depression and boiling point elevation depends on factors such as the number of particles per substrate molecule (phantom factor 'i'), the amount of substrate added to the water (molality 'm'), and water's boiling point or vapor pressure constants.
How do colligative properties formulas help in understanding vapor pressure depression and boiling point elevation?
-Colligative properties formulas provide mathematical relationships that help determine the changes in boiling point and vapor pressure due to the presence of solutes in a solution, allowing for the prediction and calculation of these changes.
What is the significance of the vapor pressure of water being equal to one atmosphere at its boiling point?
-When the vapor pressure of water is equal to one atmosphere, it signifies that the water molecules can vaporize and rise into the atmosphere, coexisting with atmospheric gases, which is a condition for boiling to occur.
How does the presence of particles in water affect its vaporization process?
-The presence of particles in water can hinder the vaporization process by increasing intermolecular forces, which requires additional heat to break these forces and allow the water to vaporize.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

13.2 Colligative Properties of Solutions (1/2)

SIFAT KOLIGATIF LARUTAN : PENURUNAN TEKANAN UAP

الخواص الجامعة للمحاليل كيمياء ثالث ثانوي 1445

Química Simples #12 - Resumos - Propriedades Coligativas

Propriedades Coligativas - Brasil Escola
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
5.0 / 5 (0 votes)
