Propriedades Coligativas - Brasil Escola
Summary
TLDRIn this educational video, Professor Choven explains the concept of colligative properties, focusing on how the addition of a non-volatile solute alters the properties of a solution. He covers four key colligative properties: tonometry (lowering of vapor pressure), ebullioscopy (increasing boiling point), cryoscopy (lowering freezing point), and osmosis (movement of solvent from less concentrated to more concentrated areas). The examples given include everyday scenarios, like making coffee or cooling drinks with salt, to illustrate the principles of each property. The video aims to provide a foundational understanding for further learning and application in calculations.
Takeaways
- 😀 Colligative properties are properties that change when a non-volatile solute is added to a solvent, and the changes depend on the quantity of solute particles, not their nature.
- 😀 The concept of vapor pressure is important in understanding colligative properties. It refers to the maximum pressure that vapor exerts over a liquid when in equilibrium.
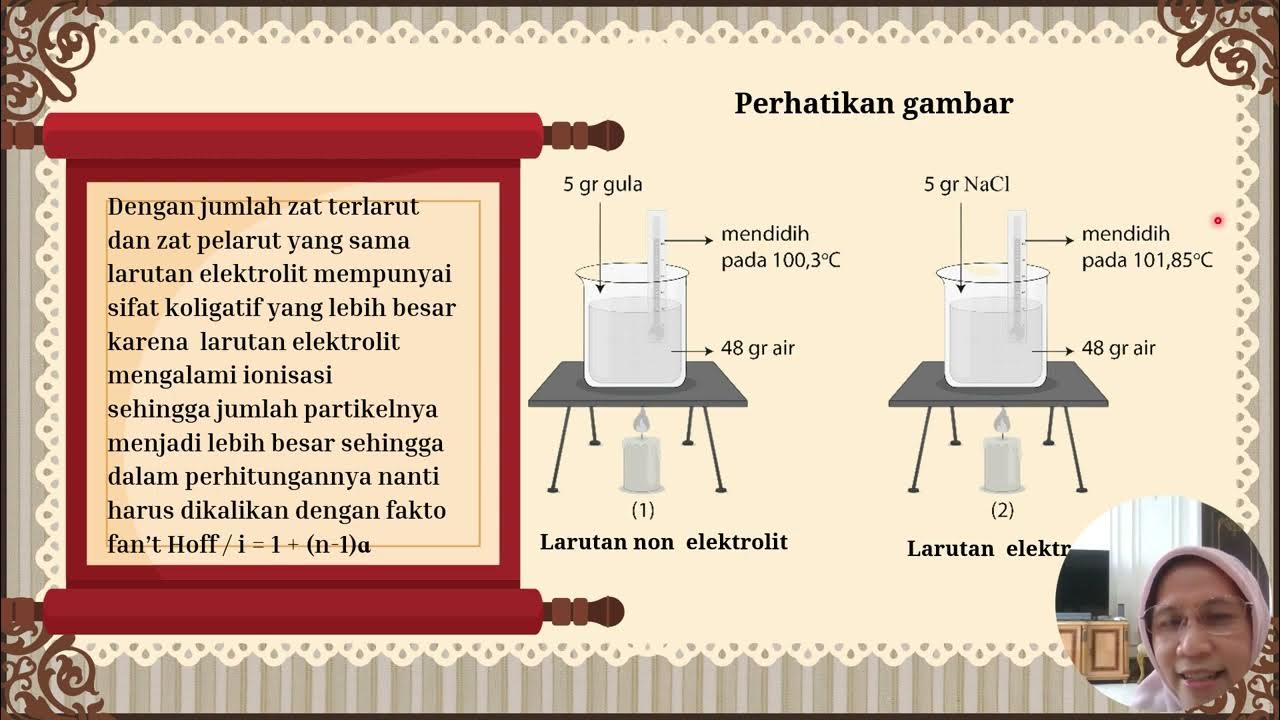
- 😀 Tonoscopia (vapor pressure lowering) occurs when a non-volatile solute is added to a solvent, decreasing the solvent's vapor pressure.
- 😀 An example of tonoscopia: Adding sugar to water lowers the vapor pressure of the water compared to pure water, which evaporates more easily.
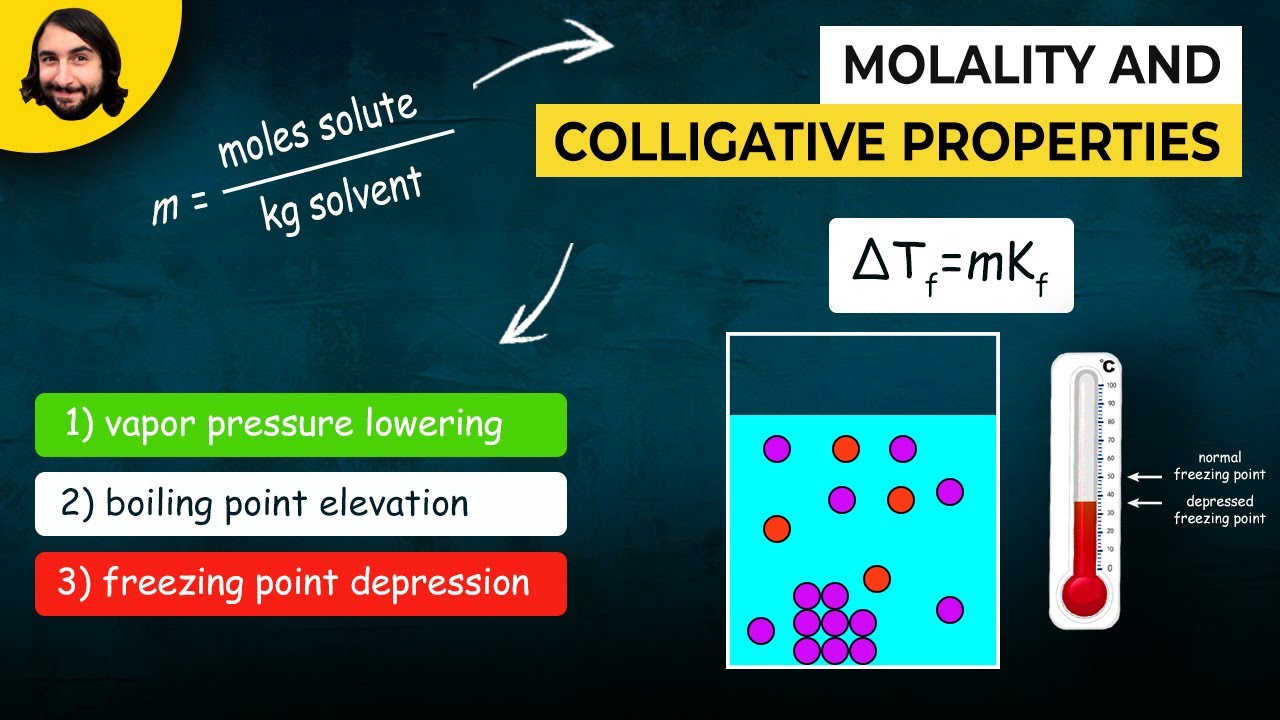
- 😀 Ebuliometria (boiling point elevation) is the increase in the boiling point of a solvent when a non-volatile solute is added.
- 😀 Examples of ebuliometria include coffee making (where adding coffee grounds increases the boiling point) and car radiators (where adding antifreeze raises the boiling point of the coolant).
- 😀 Criometria (freezing point depression) is the lowering of a solvent’s freezing point when a non-volatile solute is added.
- 😀 A practical example of criometria: Adding salt to ice speeds up the cooling process, as it lowers the freezing point of the ice.
- 😀 Osmosis is the movement of a solvent from a less concentrated solution to a more concentrated one.
- 😀 In osmotic processes, water moves from areas of lower solute concentration to areas of higher solute concentration, as shown in the example of an alfalfa leaf in water and saltwater.
- 😀 Colligative properties are crucial in practical applications like cooling beverages, preventing ice from sticking in cold weather, and enhancing vehicle engine cooling systems.
Q & A
What are colligative properties?
-Colligative properties are properties of solutions that are altered by the addition of a non-volatile solute. These properties depend on the number of solute particles, not the nature of the solute itself.
What is vapor pressure, and why is it important for colligative properties?
-Vapor pressure is the maximum pressure exerted by a vapor in equilibrium with its liquid. It's important for colligative properties because changes in vapor pressure, caused by the addition of non-volatile solutes, influence properties like boiling and freezing points.
How does tonometry affect the vapor pressure of a solvent?
-Tonometry (or tonoscopia) refers to the decrease in the vapor pressure of a solvent when a non-volatile solute is added. This lowers the solvent's tendency to evaporate.
What is an example of tonometry in real life?
-An example of tonometry is mixing water with sugar. The water with sugar evaporates less easily than pure water because the addition of sugar decreases the vapor pressure.
What does ebullioscopy (or ebuliometry) refer to?
-Ebullioscopy refers to the phenomenon where the boiling point of a solvent increases upon the addition of a non-volatile solute.
Can you provide a real-world example of ebullioscopy?
-Yes, when making coffee, adding coffee grounds to boiling water increases the boiling point of the water. Similarly, a car radiator uses a coolant with a higher boiling point to prevent the water from evaporating under high engine temperatures.
What is cryoscopy (or criometria), and how does it work?
-Cryoscopy is the lowering of the freezing point of a solvent when a non-volatile solute is added. This is due to the solute disrupting the formation of the solvent's solid structure.
How can cryoscopy be used practically?
-An example of cryoscopy is when you add salt to ice at a barbecue to cool beverages faster. The salt lowers the freezing point of the ice, making it melt quicker and cool the drink faster.
What is osmotic pressure, and how does it work?
-Osmosis is the movement of solvent from a less concentrated solution to a more concentrated solution. It occurs through a semipermeable membrane, aiming to equalize the concentrations on both sides.
What is an example of osmosis in everyday life?
-A real-life example of osmosis is when you place lettuce in water; the water moves into the cells of the lettuce, making it crisp. Conversely, if you add salt to the water, the water from the lettuce moves out, causing it to wilt.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)