SIFAT KOLIGATIF LARUTAN : PENURUNAN TEKANAN UAP
Summary
TLDRThis video explores the colligative properties of solutions, which are properties that depend on solute concentration rather than the type of solute. Key properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. The video explains these concepts through real-life examples like snow melting, boiling soup, and ice storage. It dives into the relationship between solute concentration, electrolyte behavior, and the resulting effects on vapor pressure. Using formulas and examples, it demonstrates how various solutes affect vapor pressure, including non-electrolytes and electrolytes, and explains the van't Hoff factor for accurate calculations.
Takeaways
- 😀 Colligative properties of solutions depend on solute concentration, not its type.
- 😀 Common examples of colligative properties include road salt use, cooking vegetables, and ice preservation.
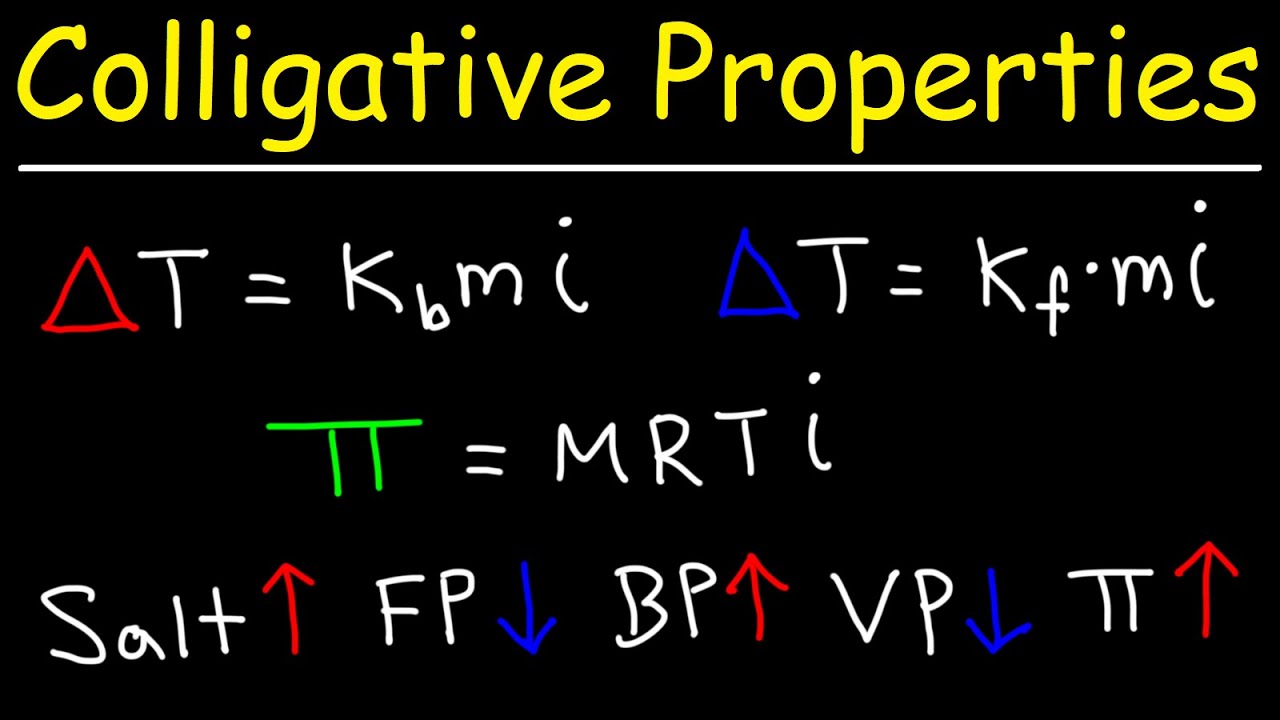
- 😀 Colligative properties include lowering vapor pressure, increasing boiling point, lowering freezing point, and osmotic pressure.
- 😀 Solution concentration can be expressed using molarity, molality, and mole fraction.
- 😀 When a solute is added to a solvent, it lowers the solvent’s vapor pressure by hindering evaporation.
- 😀 Raoult’s law explains the relationship between solute concentration and vapor pressure decrease.
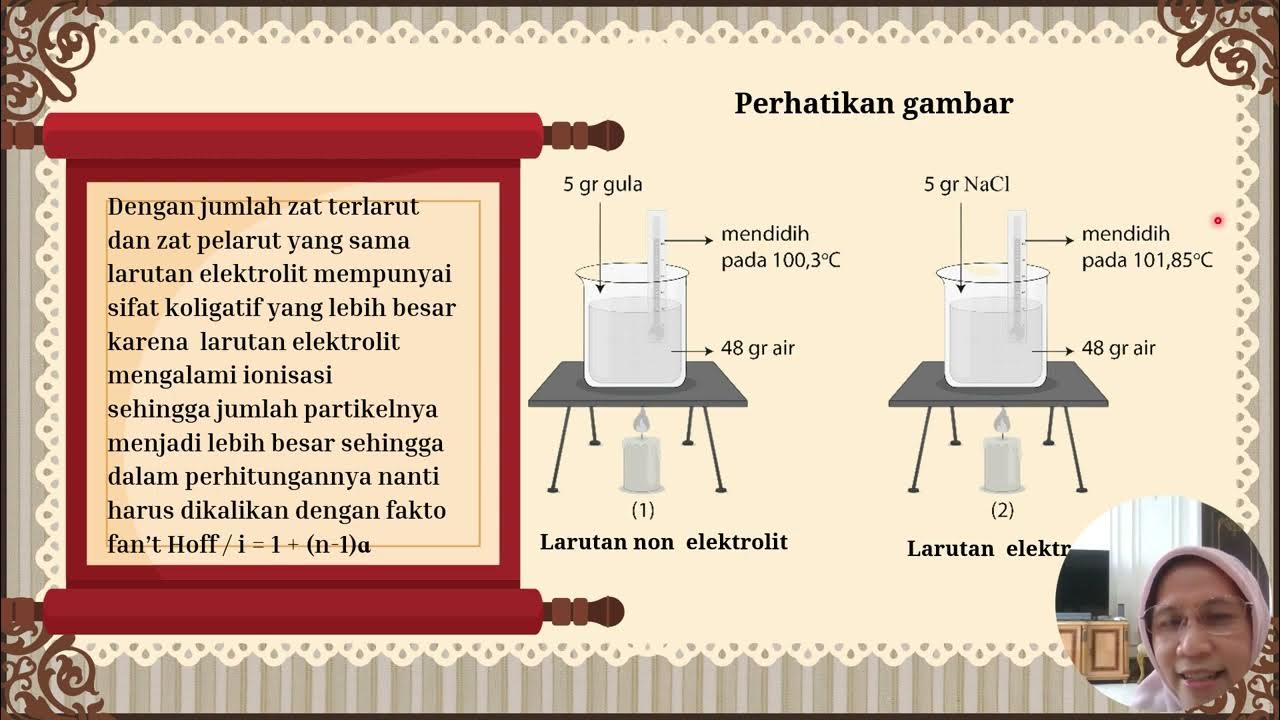
- 😀 Strong electrolytes, weak electrolytes, and non-electrolytes behave differently when dissolved in a solvent.
- 😀 A strong electrolyte like NaCl dissociates completely into ions, causing a greater reduction in vapor pressure.
- 😀 The more ions a solute produces, the greater the vapor pressure decrease in a solution.
- 😀 For electrolyte solutions, the van't Hoff factor accounts for the number of ions produced and affects calculations for vapor pressure decrease.
Q & A
What are colligative properties of solutions?
-Colligative properties of solutions are properties that depend on the concentration of solute particles but not on the type of solute. These properties include lowering vapor pressure, increasing boiling point, lowering freezing point, and osmotic pressure.
How does adding salt to the road help in snowy conditions?
-Adding salt like CaCl2 to the road in snowy conditions helps to lower the freezing point of water, preventing the formation of ice, which makes it safer for road users.
Why does the boiling of vegetable soup stop when vegetables are added?
-When vegetables are added to the soup, the boiling process stops because the addition of solutes (like the vegetable matter) reduces the vapor pressure of the solution, lowering its boiling point.
What happens when ice sellers sprinkle salt on ice?
-Ice sellers sprinkle salt on ice to lower its freezing point, which helps to maintain the ice's solid state for longer periods by preventing it from melting prematurely.
What is vapor pressure, and how does it relate to colligative properties?
-Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid. When a solute is added to a solvent, it reduces the vapor pressure of the solution, which is a key colligative property.
What effect does adding a solute like sugar have on the vapor pressure of water?
-Adding a solute like sugar to water lowers the vapor pressure because the solute molecules prevent water molecules from escaping into the vapor phase.
What is Raoult's law, and how does it relate to vapor pressure decrease?
-Raoult's law states that the vapor pressure of a solution is directly proportional to the mole fraction of the solvent. The addition of a solute lowers the vapor pressure, and this decrease can be calculated using the law.
How does the type of solute (electrolyte vs. non-electrolyte) affect the vapor pressure of a solution?
-Electrolytes dissociate into ions, increasing the number of solute particles and lowering the vapor pressure more than non-electrolytes, which do not dissociate into ions.
What is the Van't Hoff factor, and how does it affect vapor pressure calculations?
-The Van't Hoff factor (i) accounts for the number of particles produced by a solute in solution. For electrolytes, this factor helps in adjusting vapor pressure calculations, as more ions lead to a greater decrease in vapor pressure.
How do you calculate the decrease in vapor pressure for a solution?
-The decrease in vapor pressure (ΔP) is calculated using the formula ΔP = P₀ * (mol of solute / (mol of solute + mol of solvent)), where P₀ is the vapor pressure of the pure solvent.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

13.2 Colligative Properties of Solutions (1/2)
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
Sifat Koligatif 2Penurunan Tekanan Uap

ruangbelajar - Kimia XII SMA - Pendahuluan Sifat Koligatif

Colligative Properties Explained

الخواص الجامعة للمحاليل كيمياء ثالث ثانوي 1445
5.0 / 5 (0 votes)