Introduction to Fourier Transform Infrared Spectroscopy (FTIR)
Summary
TLDRIn this video, Tawanda Zimudzi introduces Fourier Transform Infrared Spectroscopy (FTIR), explaining its basis on the interaction between infrared radiation and matter. FTIR measures the frequency and intensity of absorbed infrared radiation to identify molecular bond vibrations. It offers qualitative and quantitative analysis, with applications in identifying functional groups, impurities, and polymer characterization. Advantages include speed, minimal sample prep, and non-destructive analysis, but it requires careful interpretation and calibration for quantitative results.
Takeaways
- 🌐 **FTIR Overview**: Fourier Transform Infrared Spectroscopy (FTIR) is a technique that studies the interaction between infrared radiation and matter.
- 🔍 **Infrared Interaction**: When IR light hits a sample, it can be reflected, transmitted, or absorbed, with the goal of measuring these to understand the frequency and intensity of absorbed IR radiation.
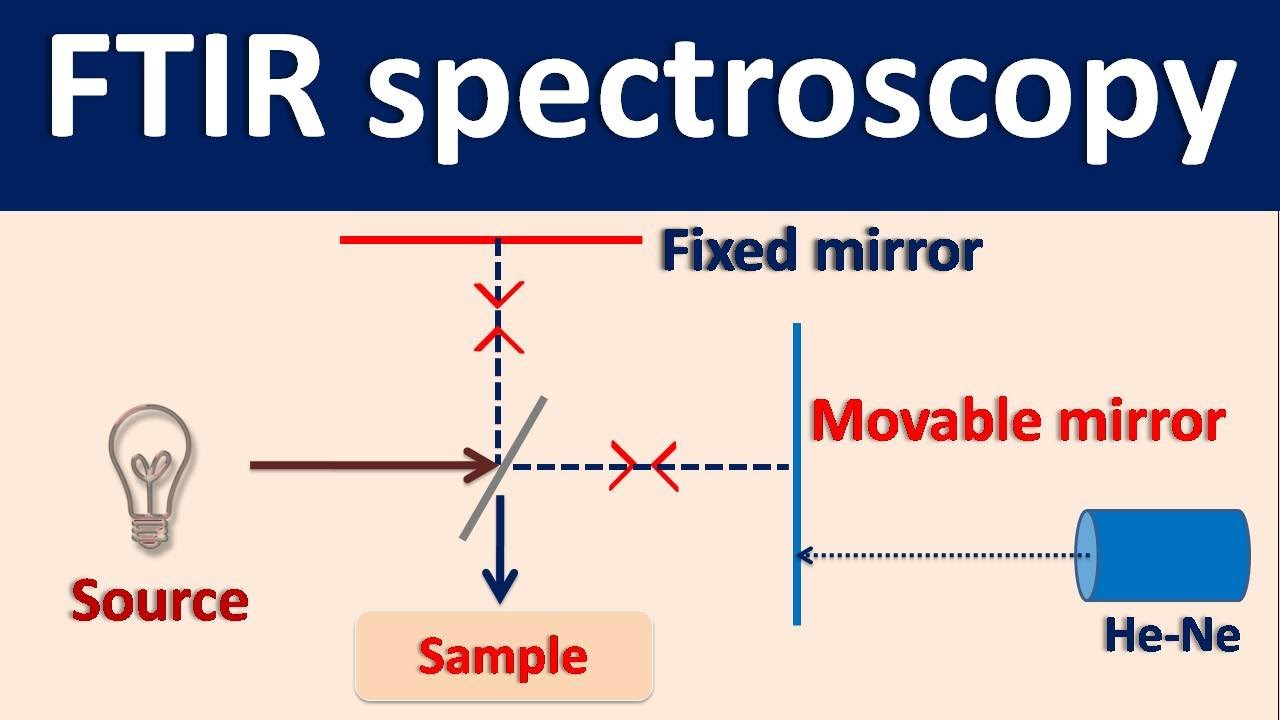
- 🔬 **Sample Analysis**: The process involves placing a sample in the path of an IR source and measuring the reflected or transmitted beam at a detector.
- 📊 **Data Presentation**: Historically, FTIR data is plotted with wave number on the X-axis and either transmittance or absorbance on the Y-axis, showing characteristic peaks or dips.
- 🔑 **Qualitative Analysis**: Qualitative information is derived from the frequency of bond vibrations, influenced by bond strength and reduced mass.
- 🔍 **Structural Information**: The 'Fingerprint Region' below 1800 inverse centimeters often provides detailed structural information, despite peak overlap complicating interpretation.
- 📈 **Quantitative Analysis**: The Beer-Lambert Equation relates absorbance to concentration, path length, and molar absorptivity, allowing for quantitative analysis.
- 🚀 **Advantages of FTIR**: FTIR is fast, requires minimal sample preparation, is adaptable for in-situ applications, non-destructive, and can be surface-sensitive.
- ⚠️ **Disadvantages of FTIR**: Spectra interpretation can be complex, true quantitative analysis challenging, and it's sensitive to CO2 and water vapor.
- 🧪 **Applications**: FTIR is used for identifying functional groups, structure elucidation, impurity determination, orientation, catalysis, process monitoring, and polymer characterization.
- 🔬 **Instrument Capabilities**: At the MCL, they have instruments covering near-IR, mid-IR, and far-IR, with capabilities for in-situ analysis, atmosphere control, and micro-analysis.
Q & A
What is Fourier Transform Infrared Spectroscopy (FTIR)?
-FTIR is a spectroscopic technique that measures the interaction between infrared radiation and matter. It involves analyzing the reflected or transmitted beam after it passes through a sample to determine the frequency and intensity of infrared radiation absorbed by the sample.
How does infrared radiation interact with a sample in FTIR?
-When an infrared beam strikes a sample, it can be reflected, transmitted, or absorbed. The goal is to measure the reflected or transmitted beam to obtain information about the frequencies and intensities of the absorbed radiation.
What is the purpose of placing a sample in the path of an IR source?
-Placing a sample in the path of an IR source allows the measurement of the reflected or transmitted beam at an infrared detector, which provides information about the molecular bond vibrations in the sample.
How does the energy absorbed by a sample relate to the transmitted beam?
-The energy absorbed by the vibrating molecules is missing in the transmitted beam, which is then converted to an electrical signal at the detector, enabling the plotting of a graph of IR frequency versus intensity.
What is the significance of the wave number on the X axis in FTIR data?
-The wave number on the X axis represents the frequency of the infrared radiation absorbed by the sample, with units of inverse centimeters.
What information can be obtained from the Y axis in an FTIR plot?
-The Y axis can represent either transmittance or absorbance. Transmittance shows dips where the sample absorbs IR radiation, while absorbance shows peaks where the IR light is absorbed.
How does qualitative analysis in FTIR work?
-Qualitative analysis is based on the frequency at which bond vibrations occur, which depends on bond strength and reduced mass. This allows for the identification of different types of chemical bonds and the distinction between different species bonded to carbon.
What is the Fingerprint Region in FTIR and why is it important?
-The Fingerprint Region is the area below 1,800 inverse centimeters in an FTIR plot. It contains structural information that is often unique to a compound, making it useful for compound identification and structure elucidation.
How does the Beer-Lambert Equation relate to quantitative analysis in FTIR?
-The Beer-Lambert Equation states that the absorbance of IR light is proportional to the concentration of the absorbing species in the sample, the path length of the sample, and the molar absorptivity. This relationship allows for the determination of pseudo-quantitative information by comparing peak areas or absorbance values.
What are some advantages of using FTIR?
-FTIR is fast, requires little to no sample preparation, is adaptable for in-situ applications, non-destructive, and can be extremely surface-sensitive.
What are the disadvantages of FTIR as mentioned in the script?
-The disadvantages of FTIR include the time-consuming nature of spectrum interpretation, difficulty in true quantitative analysis, sensitivity to carbon dioxide and water vapor, and the lack of elemental information.
What are some typical applications of FTIR?
-Typical applications of FTIR include identification of functional groups, structure elucidation, determination of impurities, orientation, catalysis, process monitoring, and polymer characterization.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Back to Basics: Fourier Transform Infrared Spectroscopy
Fourier Transform IR spectroscopy (FTIR) - How it works?

What is FTIR Spectroscopy? – Technology Introduction – METTLER TOLEDO - EN

Pengantar Spektroskopi FTIR

Infrared Spectroscopy: Key Features of Organic Functional Groups // HSC Chemistry

Infrared (IR) Spectroscopy
5.0 / 5 (0 votes)
