Infrared Spectroscopy: Key Features of Organic Functional Groups // HSC Chemistry
Summary
TLDRThis video provides a comprehensive overview of infrared spectroscopy, explaining how covalent bonds interact with infrared radiation to identify functional groups and bonds in organic compounds. It covers the key principles, such as wave numbers, transmittance, and absorbance, and discusses the distinct absorption ranges for various functional groups like alcohols, alkanes, ketones, and carboxylic acids. The video also highlights the limitations of the technique, particularly in distinguishing between isomers and certain functional groups, making it clear that while IR spectroscopy is a valuable tool, it has its constraints.
Takeaways
- 😀 Infrared spectroscopy identifies bonds and functional groups by analyzing how covalent bonds absorb infrared radiation.
- 😀 Wave number, the reciprocal of the wavelength, measures how many infrared radiation cycles fit in one centimeter.
- 😀 The infrared spectrum typically displays wave number on the x-axis and transmittance or absorbance on the y-axis.
- 😀 Major absorptions on the spectrum correspond to bonds absorbing infrared radiation, while small signals may indicate contamination.
- 😀 Alkanes and alkenes show similar infrared spectra, making it difficult to distinguish between them using infrared spectroscopy.
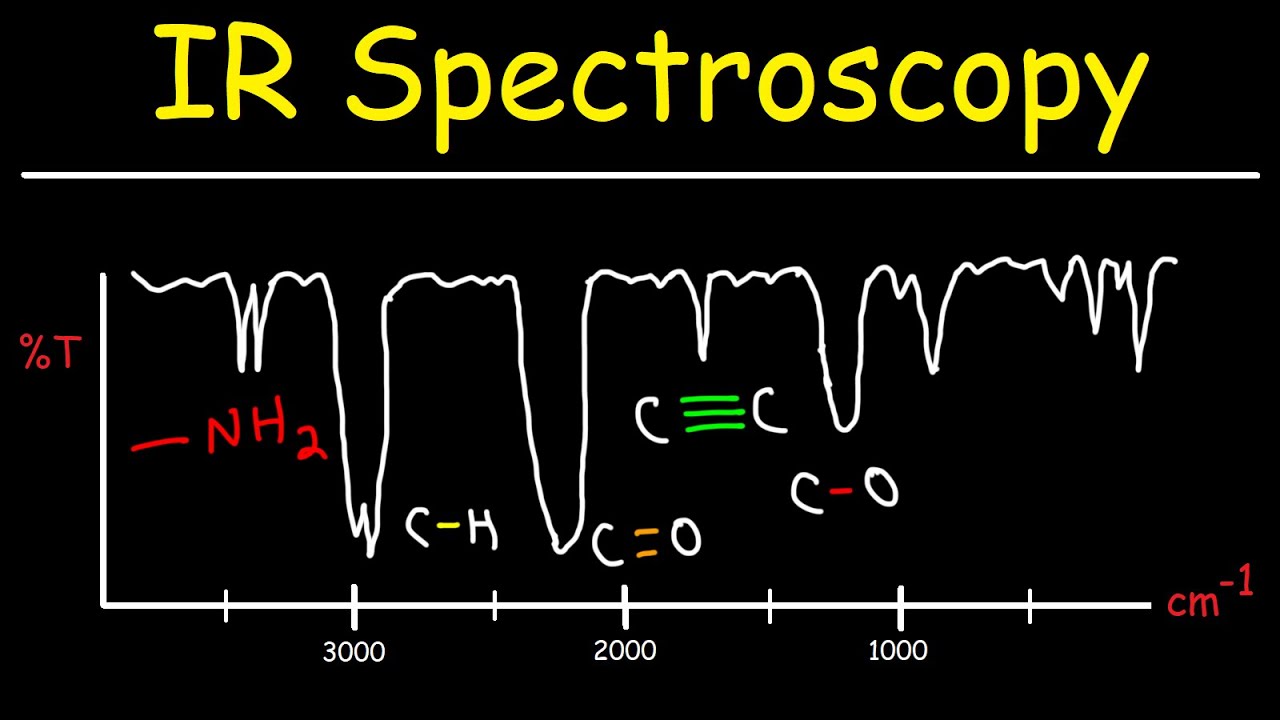
- 😀 Alcohols are easily identified by a broad absorption signal between 3230-3550 cm⁻¹, associated with the O-H bond.
- 😀 Infrared spectroscopy cannot distinguish between molecules with the same functional group, such as isomers.
- 😀 Ketones feature a characteristic carbonyl (C=O) bond absorption between 1680-1750 cm⁻¹, though this is not unique to ketones.
- 😀 Carboxylic acids can be identified by a broad O-H absorption and a C=O absorption, with the O-H absorption overlapping with the C-H signal.
- 😀 Esters are harder to identify solely through infrared spectroscopy due to a hidden C-O bond absorption in the lower wave number range.
- 😀 Organic bases like amines show a distinctive N-H bond absorption between 3300-3500 cm⁻¹, different from O-H bonds in alcohols and acids.
- 😀 Nitrile groups have a unique C≡N bond absorption between 2220-2260 cm⁻¹, making them easily identifiable in the infrared spectrum.
Q & A
What is the basic principle behind infrared spectroscopy?
-Infrared spectroscopy relies on the interaction between covalent bonds and infrared radiation. When covalent bonds absorb infrared radiation, they vibrate, and this interaction helps identify different bonds and functional groups in organic compounds.
What does the wave number in infrared spectroscopy represent?
-The wave number in infrared spectroscopy is a measurement of how many wavelengths of infrared radiation can pass through in one centimeter. It is the reciprocal of the wavelength and is used to identify specific bonds based on the energy they absorb.
How is the infrared spectrum oriented on the graph?
-In an infrared spectrum, the x-axis represents the wave number of infrared radiation, with higher wave numbers on the left and lower wave numbers on the right. The y-axis is typically labeled as transmittance, indicating the percentage of radiation passing through the sample.
What does a major absorption dip in an infrared spectrum signify?
-A major absorption dip in the infrared spectrum indicates that a specific wave number of infrared radiation has been completely absorbed by the sample, meaning that the corresponding covalent bond has vibrated due to the absorbed energy.
Why is infrared spectroscopy not particularly useful for distinguishing between alkenes and alkanes?
-Infrared spectroscopy is not very useful for distinguishing between alkenes and alkanes because both functional groups contain similar bonds, such as CH and CC bonds. The presence of a CC double bond in alkenes is often hard to distinguish in the spectrum due to its narrow and small absorption.
How can alcohols be identified in infrared spectroscopy?
-Alcohols can be identified in infrared spectroscopy due to the presence of a broad absorption signal caused by the oxygen-hydrogen (OH) bond, which typically appears between 3230 and 3550 cm-1.
What is the limitation of infrared spectroscopy when it comes to distinguishing isomers?
-Infrared spectroscopy is not effective at distinguishing isomers because isomers typically contain the same types of covalent bonds, leading to very similar infrared spectra, even if the molecules are structurally different.
What is a characteristic feature of a ketone in infrared spectroscopy?
-Ketones can be identified in infrared spectroscopy by the presence of a prominent absorption signal due to the carbonyl (C=O) group, typically found in the range of 1680 to 1750 cm-1.
How do carboxylic acids differ from alcohols in terms of their infrared absorption?
-Carboxylic acids have a much broader absorption signal due to the OH bond, which overlaps with the CH bond, and the absorption occurs at a lower wave number (2500–3000 cm-1) compared to alcohols. Additionally, carboxylic acids also show a C=O double bond absorption around 1680 to 1750 cm-1.
What makes esters challenging to identify using infrared spectroscopy?
-Esters are challenging to identify in infrared spectroscopy because the C=O (carbonyl) bond is located in the middle of the spectrum, but the C–O (single bond) signal is at a much lower wave number (1,100–1,300 cm-1), which is often buried under other signals, making it difficult to identify the ester.
What distinctive feature in the infrared spectrum can help identify nitrile compounds?
-Nitrile compounds can be identified in infrared spectroscopy due to the characteristic absorption of the carbon-nitrogen (C≡N) triple bond, which occurs in the range of 2220–2260 cm-1. This is a unique wave number range not found in other covalent bonds.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

IR Spectroscopy

Introduction to Fourier Transform Infrared Spectroscopy (FTIR)
IR Spectroscopy and Mass Spectrometry: Crash Course Organic Chemistry #5
IR Spectroscopy - Basic Introduction

INFRA RED SPECTROSCOPY. How to analyze the diagram?

How to Interpret an IR Spectrum and Identify the RIGHT Functional Group
5.0 / 5 (0 votes)