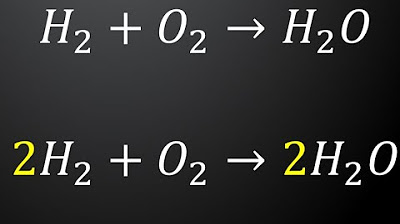Writing Chemical Equations in Words
Summary
TLDRThis educational video by Professor Dave focuses on how to write chemical equations, both in symbols and words. It explains how to translate between chemical formulas and descriptive sentences, helping learners understand the components of a chemical reaction. The video provides examples, such as the reaction between sodium and water and the formation of iron(III) fluoride, illustrating how to convert between written words and chemical symbols. The lesson also touches on balancing equations, ensuring students grasp the full process of depicting chemical reactions accurately.
Takeaways
- 😀 Understand that chemical equations can be written using both symbols (chemical formulas) and words.
- 😀 Chemical formulas abbreviate the elements present in each compound and may include phase symbols (solid, liquid, gas, aqueous).
- 😀 Writing chemical equations with words helps in verbalizing and communicating reactions more clearly.
- 😀 The transformation from symbols to words involves describing the compounds and their phases in full detail.
- 😀 For example, Na (solid) plus H2O (liquid) reacts to produce NaOH (aqueous) and H2 (gas).
- 😀 The phrase 'reacts with' indicates the reactants, while 'to produce' indicates the products in word descriptions.
- 😀 To convert a word sentence into chemical symbols, identify the elements, their states, and their stoichiometric ratios.
- 😀 For example, 'Solid iron reacts with fluorine gas to produce solid iron(III) fluoride' becomes Fe (solid) + F2 (gas) → FeF3 (solid).
- 😀 Roman numerals in compound names (like iron(III) fluoride) indicate the oxidation state of an element.
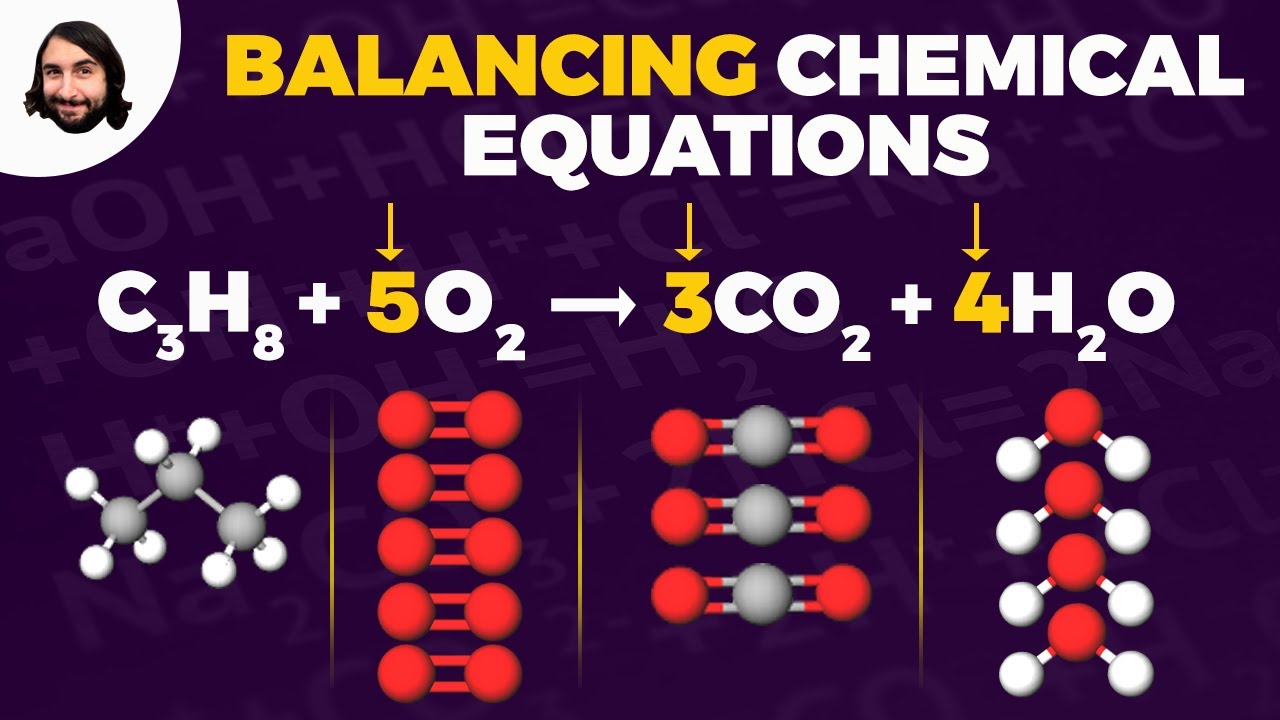
- 😀 Balancing the chemical equation is important; make sure the number of atoms on both sides of the equation are equal.
- 😀 An example of balancing: 2 Fe + 3 F2 → 2 FeF3, ensuring 6 fluorines and 2 irons on both sides.
Q & A
Why is it important to be able to write chemical equations in both symbols and words?
-Writing chemical equations in both symbols and words helps to better understand and communicate chemical reactions. Using words can provide clearer context, while symbols offer a more concise and universally recognized representation.
What is the role of chemical formulas in writing chemical equations?
-Chemical formulas abbreviate the elements present in compounds, making equations more concise. They also sometimes indicate the phase of each compound, helping to convey more detailed information about the reaction.
How do we translate a symbolic chemical equation into a word equation?
-To translate a symbolic equation into words, you replace the chemical symbols with their corresponding element names, describe the phases of matter (solid, liquid, gas, aqueous), and use words like 'reacts with' or 'produces' to describe the process.
What key phrase in a word equation signals the transition from reactants to products?
-The phrase 'to produce' signals the transition from reactants to products in a word equation.
In the given example, what is the word equation for the reaction between sodium and water?
-The word equation is: 'Solid sodium metal reacts with liquid water to produce aqueous sodium hydroxide and hydrogen gas.'
How do we convert a word equation into a chemical equation?
-To convert a word equation into a chemical equation, identify the chemical symbols for each element and compound, represent the phases (solid, liquid, gas, aqueous), and use an arrow to separate reactants from products.
What does the Roman numeral in 'iron(III) fluoride' tell us about the compound?
-The Roman numeral 'III' indicates that the iron has a +3 oxidation state, meaning each iron ion is paired with three fluoride ions (each with a -1 charge) to form a neutral compound.
How do we balance a chemical equation, such as the one involving iron and fluorine?
-To balance the equation, ensure that the number of atoms for each element is the same on both sides. In the example, adjust the coefficients of iron and fluorine to balance the number of atoms, resulting in '2Fe + 3F₂ → 2FeF₃'.
What is the significance of phase symbols in chemical equations?
-Phase symbols (s for solid, l for liquid, g for gas, and aq for aqueous) provide important information about the physical state of the compounds involved in the reaction, which can influence the reaction conditions and products.
Why do we sometimes use the word 'reacts with' in a chemical equation?
-'Reacts with' is used to describe the interaction between reactants in a chemical equation. It helps to indicate which substances are involved in the reaction and how they combine to form new products.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)