Chemistry: Balancing Chemical Equations (Tagalog Explained)
Summary
TLDRIn this educational video, viewers are guided through the process of balancing chemical equations, a fundamental skill in chemistry. The instructor begins by explaining the components of a chemical equation, including reactants, products, chemical symbols, subscripts, and coefficients. The video then delves into the importance of ensuring equal atom counts for each element in the equation. Through step-by-step examples, the instructor demonstrates how to balance equations by adjusting coefficients while adhering to the rules of chemistry. The video is a valuable resource for students looking to master chemical equation balancing.
Takeaways
- 🔬 The video focuses on balancing chemical equations, a fundamental skill in chemistry.
- 📚 It introduces the parts of a chemical equation, including reactants, products, and their chemical symbols.
- ✏️ The importance of subscripts in chemical formulas is highlighted, which indicate the number of atoms of each element.
- 🔢 Coefficients, which represent the number of molecules, are explained, with examples showing how they multiply with subscripts to give the total number of atoms.
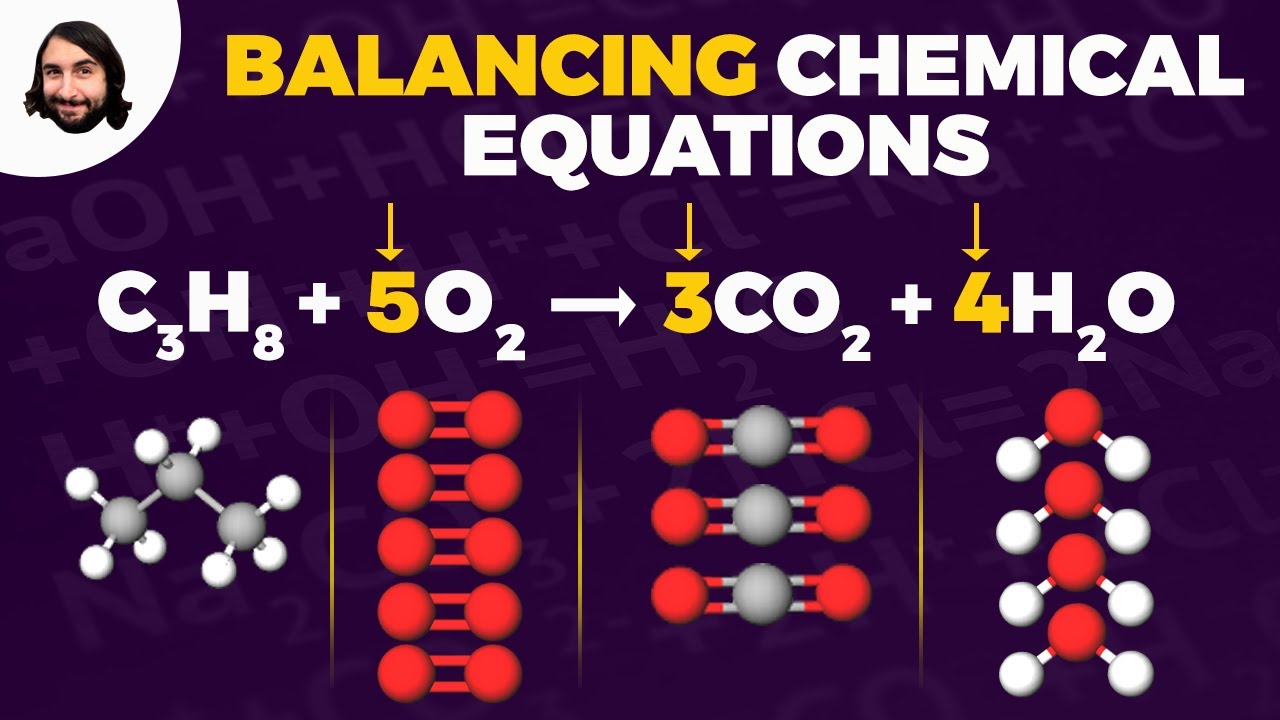
- 🧪 The video walks through the process of balancing equations by ensuring equal numbers of atoms for each element on both sides of the equation.
- 📝 Rules for balancing chemical equations are outlined, emphasizing that only coefficients can be adjusted and that subscripts or parentheses should not be altered.
- 🌟 Practical examples are provided, demonstrating how to balance various types of chemical equations step by step.
- 📈 The video uses a systematic approach to balance equations, starting with the most complex molecules and working towards simpler ones.
- 📊 A table format is used to organize reactants and products, facilitating the comparison and balancing of atoms.
- 👨🏫 The presenter, James, is a math and language instructor who aims to make chemistry more accessible through clear explanations and examples.
- 🎓 The video concludes with a teaser for the next topic, molar mass, encouraging viewers to stay tuned for further educational content.
Q & A
What are the main components of a chemical equation?
-The main components of a chemical equation include reactants, products, chemical symbols, subscripts, coefficients, and the equal sign that connects them.
What is the role of subscripts in a chemical equation?
-Subscripts indicate the number of atoms of a particular element within a molecule or compound.
How do coefficients in a chemical equation relate to the number of molecules?
-Coefficients represent the number of molecules or formula units of a substance involved in a chemical reaction.
Why is it important to balance the number of atoms for each element in a chemical equation?
-Balancing the number of atoms ensures that the law of conservation of mass is followed, meaning the number of atoms of each element is the same before and after the reaction.
What is the first rule mentioned in the script for balancing chemical equations?
-The first rule mentioned is that you may only add coefficients when balancing chemical equations.
Can you change subscripts or add parentheses to balance a chemical equation?
-No, according to the script, you should never add subscripts or parentheses or anything other than coefficients to a chemical equation to balance it.
What does the law of conservation of mass mean in the context of chemical equations?
-The law of conservation of mass means that in a chemical reaction, the total mass of the reactants equals the total mass of the products, and the number of atoms of each element remains constant.
How do you calculate the total number of atoms for an element in a chemical equation?
-You multiply the coefficient of a substance by the subscript of the element in the chemical formula to get the total number of atoms for that element.
What is an example of a chemical equation provided in the script?
-An example given is '2H2 + O2 = 2H2O', which represents the reaction of hydrogen gas with oxygen gas to form water.
What is the purpose of constructing a table of reactants and products when balancing chemical equations?
-Constructing a table helps to organize and compare the number of atoms of each element on both sides of the equation to ensure they are balanced.
How does changing the coefficient of a compound affect the balance of a chemical equation?
-Changing the coefficient of a compound affects the number of molecules of that compound in the reaction, which in turn affects the number of atoms of each element, requiring adjustments to balance the equation.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Persamaan reaksi dan penyetaraan reaksi kimia - Kimia SMA kelas 10 semester 2
Balancing Chemical Equations

Penyetaraan Persamaan Reaksi Kimia dengan Cepat- Kimia Kelas 10

PERSAMAAN REAKSI DAN CARA PENYETARAANNYA ( KIMIA SMA KELAS 10 )
How to Write Chemical Equations

Introduction to Balancing Chemical Equations
5.0 / 5 (0 votes)