Balancing Chemical Equations
Summary
TLDRIn this tutorial, Professor Dave explains how to balance chemical equations, a vital skill in chemistry. He introduces the concept of reactants, products, and the role of coefficients and subscripts in equations. The process of balancing involves ensuring the same number of atoms on both sides, with elements balanced one by one. Using examples like the reaction of sodium and chlorine to form sodium chloride, and the combustion of propane, the video guides viewers through the steps of balancing equations effectively. This methodical approach is essential for understanding and predicting chemical reactions.
Takeaways
- 😀 Chemical equations represent the interaction between substances during a reaction, showing reactants and products.
- 😀 The arrow in a chemical equation separates reactants (the substances that react) from products (the substances that are produced).
- 😀 To be accurate, chemical equations need to be balanced to ensure the same number of atoms on both sides, following the law of conservation of mass.
- 😀 Subscripts in a chemical equation indicate the number of atoms of an element in one molecule.
- 😀 Coefficients in a chemical equation indicate how many molecules of a substance are involved in the reaction.
- 😀 Chlorine gas (Cl₂) consists of two chlorine atoms per molecule, so it reacts with two sodium atoms to form sodium chloride (NaCl).
- 😀 In the reaction of hydrogen gas and oxygen gas to form water (H₂O), balancing requires adjusting the coefficients for hydrogen and oxygen.
- 😀 When balancing equations, start with elements that appear in only one compound on both sides of the equation.
- 😀 The balancing process involves adjusting coefficients to match the number of atoms of each element on both sides of the equation.
- 😀 Using the example of propane (C₃H₈), balance carbon atoms first, then hydrogen, and finally oxygen to achieve a balanced equation.
- 😀 The final balanced equation for the combustion of propane is C₃H₈ + 5O₂ → 3CO₂ + 4H₂O, where all elements are accounted for.
Q & A
What is a chemical equation?
-A chemical equation is a way to describe a chemical reaction, showing the reactants (the substances that react) and the products (the substances that are formed) along with the direction of the reaction.
What is the role of the arrow in a chemical equation?
-The arrow in a chemical equation separates the reactants from the products and indicates the direction of the reaction.
Why do we need to balance chemical equations?
-Balancing chemical equations ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the law of conservation of mass, which states that atoms cannot be created or destroyed.
What is the difference between subscripts and coefficients in a chemical equation?
-Subscripts tell you how many atoms of a specific element are in one molecule of a substance, while coefficients indicate how many molecules of that substance are involved in the reaction.
Why does chlorine gas need two atoms in a molecule?
-Chlorine gas (Cl₂) consists of diatomic molecules, meaning each chlorine molecule is made up of two chlorine atoms bound together.
How do we balance an equation like the one involving sodium and chlorine?
-To balance the sodium-chlorine equation, we use coefficients: one molecule of chlorine gas reacts with two sodium atoms to form two molecules of sodium chloride, ensuring equal numbers of chlorine and sodium atoms on both sides.
What is the step-by-step method for balancing chemical equations?
-First, balance the elements that are only present in one compound on each side of the equation. Then balance elements that appear in multiple compounds. Start with elements like carbon, then hydrogen, and finally oxygen, adjusting the coefficients as needed.
In the propane combustion reaction, why did we put a coefficient of 3 in front of CO₂?
-We placed a coefficient of 3 in front of CO₂ to balance the number of carbon atoms. Since there are 3 carbon atoms in propane (C₃H₈), we need 3 molecules of carbon dioxide (CO₂) to ensure the carbon atoms are balanced on both sides.
How do we balance hydrogen in the propane combustion equation?
-To balance hydrogen, we adjust the coefficient in front of water (H₂O). Since there are 8 hydrogen atoms in propane, we need 4 water molecules (4 × 2 = 8 hydrogen atoms) to balance the hydrogen atoms on both sides.
Why do we need 5 molecules of O₂ in the propane combustion reaction?
-The right side of the equation has 6 oxygen atoms from CO₂ and 4 oxygen atoms from H₂O, totaling 10 oxygen atoms. To balance this, we need 5 molecules of O₂ (5 × 2 = 10 oxygen atoms) on the left side.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Penyetaraan Persamaan Reaksi Kimia dengan Cepat- Kimia Kelas 10

Solution Chemistry and Net Ionic Equations
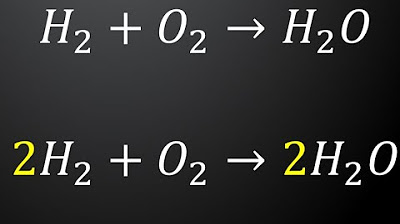
Chemistry: Balancing Chemical Equations (Tagalog Explained)

Writing Chemical Equations in Words

Balanceamento de Equações Químicas - Brasil Escola

Video Pembelajaran Model Problem Based Learning Materi Persamaan Reaksi Kimia
5.0 / 5 (0 votes)