What are Electrons and Excitation?
Summary
TLDRThe video explains how electrons move within atoms, changing energy levels when excited. In fluorescent bulbs, electrons collide with mercury vapor, causing electrons to shift to higher energy states. As they return to lower states, ultraviolet photons are emitted, which excite phosphor electrons. This repeated excitation and de-excitation process releases photons that create visible white light. The process is essential to how fluorescent lightbulbs function, with an electric current driving the movement of electrons and producing the characteristic glow of white light.
Takeaways
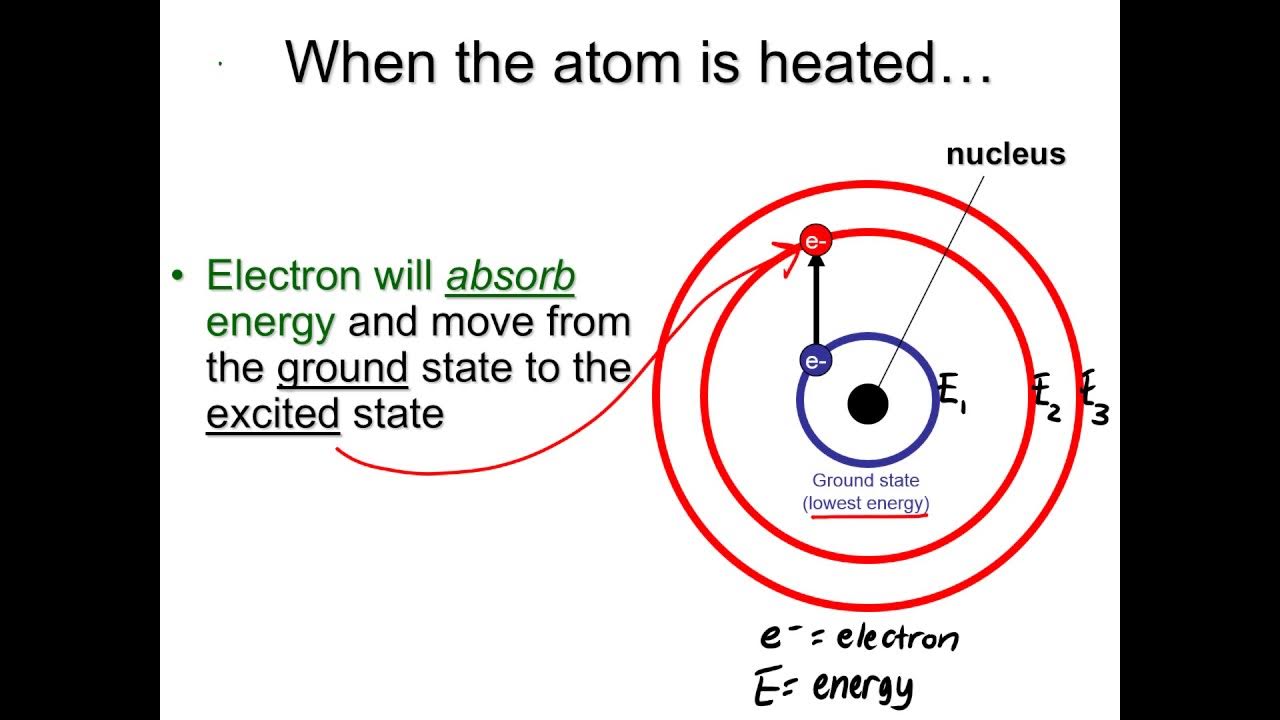
- 🔬 Electrons orbit the nucleus of an atom in concentric energy levels.
- 🌌 The farther an electron is from the nucleus, the higher its potential energy.
- 💥 Electrons can be excited to a higher energy level by colliding with free electrons.
- 🌀 Excited electrons quickly return to their original energy level, emitting light in the process.
- 💡 The principle of electron excitation and de-excitation powers fluorescent lights.
- 🌫️ Fluorescent bulbs contain argon gas and a small amount of vaporized mercury.
- 💡 The inner surface of the tube is coated with phosphor to convert ultraviolet light to visible light.
- 🔌 Electrodes at each end of the tube emit electrons when heated.
- ⚡ AC voltage from a starter pushes electrons through the tube, causing collisions with mercury vapor electrons.
- 🌐 The absorbed ultraviolet photons by phosphor excite electrons, which then release visible light photons.
- 💡 The combined effect of these reactions produces the characteristic white glow of fluorescent bulbs.
Q & A
What are the orbits or shells around an atom's nucleus?
-The orbits or shells around an atom's nucleus represent different energy states that electrons can occupy.
How does an electron's distance from the nucleus affect its energy?
-The farther an electron is from the nucleus, the greater its potential energy.
What happens when an electron is knocked to a higher energy level?
-When an electron is knocked to a higher energy level, it enters a state of excitation, but this state is temporary, and the electron quickly returns to its original orbit, releasing a photon in the process.
How is visible light produced in the process of electron de-excitation?
-Visible light is produced when an electron returns from a higher energy level to a lower one, emitting a photon of visible light during this transition.
What is the main gas used inside a fluorescent lightbulb?
-A fluorescent lightbulb is typically filled with argon gas and a small amount of vaporized mercury.
What role does the phosphor coating inside the fluorescent bulb play?
-The phosphor coating inside the fluorescent bulb absorbs ultraviolet photons, which excites its electrons, and as these electrons return to their normal state, they release photons that contribute to the bulb's visible white light.
How do the electrodes in a fluorescent bulb function?
-The electrodes in a fluorescent bulb contain filaments that, when heated to a high temperature, emit or boil off electrons, which are then pushed across the tube by an AC voltage pulse.
What happens when a free electron collides with a mercury vapor electron in a fluorescent bulb?
-When a free electron collides with a mercury vapor electron, the mercury electron is bumped to a higher energy level. It then returns to its lower state, releasing an ultraviolet photon.
How does ultraviolet light become visible in a fluorescent bulb?
-Ultraviolet light emitted by the excited mercury vapor electrons is absorbed by the phosphor coating, which then releases visible light as the electrons return to a lower energy state.
What is the overall mechanism behind the light emission in a fluorescent bulb?
-The light emission in a fluorescent bulb is due to a repeating process where electrons are excited to higher energy levels and then de-excited, releasing photons. This process occurs both with mercury vapor and the phosphor coating, resulting in visible white light.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Flame Tests Spectra Notes

GCSE Physics - Atomic Structure, Isotopes & Electrons Shells #32

Emission and absorption spectra [IB Physics SL/HL]

Energy Levels and Spectra (Quantum Phenomena 5)

Bohr Model in Brief: The planetary model, its connection to emission spectra & quantized electrons.

Shells-Subshells-Orbitals
5.0 / 5 (0 votes)