Emission and absorption spectra [IB Physics SL/HL]
Summary
TLDRThis video discusses the emission and absorption spectra of atoms and their significance. It explains how excited electrons in atoms release photons when they drop to lower energy levels, resulting in unique emission spectra for different elements. The absorption spectrum is also explored, where gases absorb photons of specific energies, leaving dark lines in a continuous spectrum. These spectral patterns serve as 'fingerprints' for identifying elements, allowing scientists to determine the composition of stars and even exoplanet atmospheres. The video also touches on redshift in astronomy and new discoveries from the James Webb Space Telescope.
Takeaways
- 🔋 Excited atoms' electrons move up in energy levels when stimulated by an external energy source.
- 🌟 Electrons drop back down to lower energy levels, emitting photons in the process.
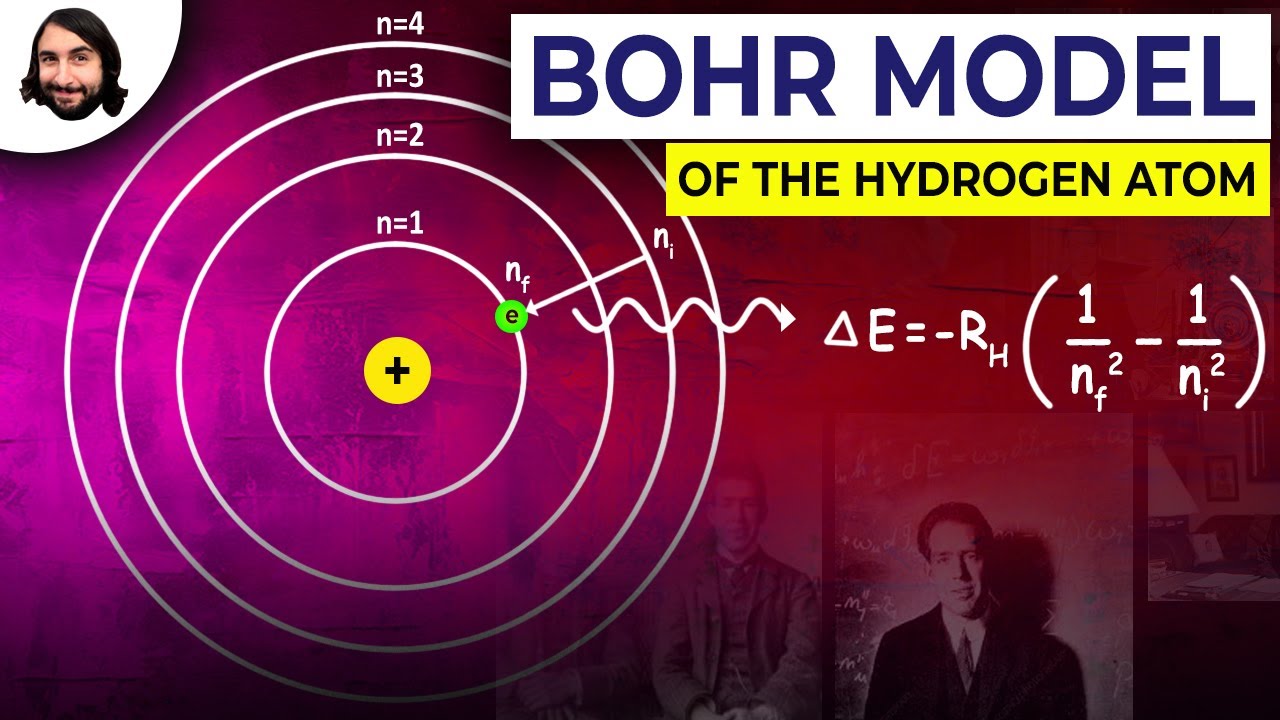
- 🔬 The energy of emitted photons is related to the frequency by E = hf, where f is the frequency.
- 🌈 Different energy levels correspond to different photon colors and wavelengths.
- 💡 Emission spectra show distinct photon frequencies based on energy drops within an atom.
- 🧬 Each element has a unique emission spectrum, acting as a fingerprint for identifying substances.
- 📉 Absorption spectra are the opposite of emission spectra, showing dark lines where certain energies are absorbed.
- 🧪 The absorption and emission lines reveal the chemical composition and atomic structure of a substance.
- 🚀 Spectral analysis can be used to study stars and distant celestial bodies, revealing their composition and movement.
- 🌍 Spectral analysis of exoplanets, such as through the James Webb Telescope, can determine their atmospheric composition, possibly hinting at signs of life.
Q & A
What is an emission spectrum?
-An emission spectrum is a pattern of light emitted by energized atoms or molecules. When electrons in atoms drop from higher energy levels to lower ones, they emit photons with specific energies, creating a unique pattern of light.
How does an absorption spectrum differ from an emission spectrum?
-An absorption spectrum is created when a continuous light source passes through a cooler gas, and the gas absorbs certain wavelengths of light. This results in a bright background with dark lines at specific frequencies, whereas an emission spectrum has a dark background with bright lines at specific frequencies.
Why are emission and absorption spectra important in understanding atomic structure?
-Emission and absorption spectra are crucial for understanding atomic structure because they reveal the discrete energy levels within atoms. The specific wavelengths of light emitted or absorbed correspond to the energy differences between these levels, providing evidence for quantized energy states.
What does the term 'quantized' mean in the context of atomic energy levels?
-In the context of atomic energy levels, 'quantized' means that energy levels are discrete and can only take on certain specific values. Electrons cannot exist in between these levels; they can only occupy these quantized energy states.
How can the emission spectrum of hydrogen be described?
-The emission spectrum of hydrogen is characterized by specific lines at wavelengths corresponding to the energy differences between its quantized energy levels. These lines are unique to hydrogen and can be used as a 'fingerprint' to identify it.
What is the significance of sodium's double lines in its absorption spectrum?
-Sodium's double lines in its absorption spectrum are particularly bright and characteristic. They indicate the presence of sodium in a sample because they correspond to specific energy transitions within sodium atoms.
How can the composition of a star be determined using spectral analysis?
-The composition of a star can be determined by analyzing the absorption lines in its spectrum. Each element has a unique pattern of lines that correspond to its atomic energy levels. By identifying these lines, astronomers can deduce which elements are present in the star's atmosphere.
What is the Doppler effect, and how does it relate to the redshift observed in distant galaxies?
-The Doppler effect is the change in frequency or wavelength of a wave in relation to an observer who is moving relative to the wave source. In astronomy, a redshift occurs when light from a distant galaxy is shifted towards the red end of the spectrum, indicating that the galaxy is moving away from us.
How can spectral analysis help in the detection of exoplanets?
-Spectral analysis can help detect exoplanets by observing the light from a star as a planet transits in front of it. The planet's atmosphere may absorb certain wavelengths of light, creating a unique absorption spectrum that can reveal the composition of the planet's atmosphere.
What is the significance of detecting oxygen or methane in the atmosphere of an exoplanet?
-Detecting oxygen or methane in an exoplanet's atmosphere could suggest the presence of life, as these gases can be byproducts of biological processes. However, they can also be produced by non-biological processes, so their detection would require further investigation to confirm the presence of life.
How does the process of spectral analysis help in understanding the composition of celestial objects?
-Spectral analysis helps in understanding the composition of celestial objects by identifying the specific wavelengths of light that are emitted or absorbed. Each element has a unique spectral fingerprint, allowing scientists to determine which elements are present in stars, galaxies, or exoplanets.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)