Shells-Subshells-Orbitals
Summary
TLDRThis video from M Math and Science explains the structure of atoms by exploring energy levels, sublevels, and orbitals where electrons reside. It covers the seven energy levels, their maximum electron capacities using the formula 2n², and the increasing energy as electrons move farther from the nucleus. The video details sublevels (s, p, d, f), their electron limits, and orbital arrangements, illustrating how electrons occupy these spaces with opposite spins according to the Pauli exclusion principle. Clear visuals help viewers understand electron configurations, making complex atomic concepts accessible and engaging, all while ending with a reminder about the power of kindness.
Takeaways
- 🔋 There are up to seven energy levels (shells) in an atom, each holding a maximum number of electrons.
- 📈 Moving to higher energy levels requires more energy and they are numbered using the principal quantum number (n).
- 🧮 The maximum number of electrons in an energy level can be calculated using the formula 2 * n².
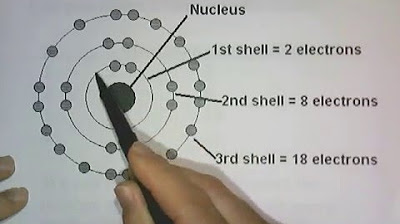
- 📊 For the first four energy levels, the maximum electrons are: Level 1 = 2, Level 2 = 8, Level 3 = 18, Level 4 = 32.
- ⚛ Within each energy level, there are sublevels labeled s, p, d, and f.
- 🪐 Each sublevel can hold a specific number of electrons: s = 2, p = 6, d = 10, f = 14.
- 🌀 Each sublevel contains orbitals: s = 1 orbital, p = 3 orbitals, d = 5 orbitals, f = 7 orbitals, and each orbital can hold 2 electrons.
- ↕ The Pauli exclusion principle states that electrons in the same orbital spin in opposite directions, represented by up and down arrows.
- 💡 Visualizing energy levels and orbitals helps understand electron arrangement in atoms.
- 💖 The video also encourages a positive message: kindness multiplies kindness.
Q & A
What are energy levels in an atom?
-Energy levels, also called principal quantum numbers (n), are regions around the nucleus where electrons can exist. They are numbered from 1 to 7, with higher numbers being farther from the nucleus and requiring more energy.
How can you calculate the maximum number of electrons in an energy level?
-The maximum number of electrons in an energy level can be calculated using the formula 2n², where n is the principal quantum number.
What is the maximum number of electrons in the first four energy levels?
-Level 1: 2 electrons, Level 2: 8 electrons, Level 3: 18 electrons, Level 4: 32 electrons.
What are sublevels, and what types exist within energy levels?
-Sublevels are divisions within energy levels that indicate different shapes of electron clouds. The types of sublevels are s, p, d, and f.
How many electrons can each sublevel hold?
-The s sublevel can hold 2 electrons, p can hold 6 electrons, d can hold 10 electrons, and f can hold 14 electrons.
What are orbitals, and how are they related to sublevels?
-Orbitals are regions within sublevels where electrons are most likely to be found. Each orbital can hold a maximum of 2 electrons. Sublevels have a specific number of orbitals: s has 1, p has 3, d has 5, and f has 7.
What is the Pauli Exclusion Principle?
-The Pauli Exclusion Principle states that no two electrons in the same orbital can have the same spin. Electrons in the same orbital must spin in opposite directions, represented by arrows ↑↓.
How do electron spins help represent electrons in orbitals?
-Electron spins are represented by arrows pointing up or down. Each orbital can contain one up-spin and one down-spin electron to satisfy the Pauli Exclusion Principle.
Why do higher energy levels require more energy for electrons?
-Electrons farther from the nucleus are less tightly bound, so moving to higher energy levels requires more energy to overcome the attraction from the positively charged nucleus.
Can you explain the hierarchy from energy levels to electrons?
-The hierarchy is as follows: Energy Level → Sublevel (s, p, d, f) → Orbital → Electron. Each level contains sublevels, sublevels contain orbitals, and orbitals contain electrons with opposite spins.
Why is it useful to visualize sublevels and orbitals?
-Visualizing sublevels and orbitals helps understand electron configurations, the distribution of electrons in an atom, and chemical behavior, including bonding and reactivity.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Energy levels, sublevels, & orbitals
Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle

3.6.3 - Como fazer a distribuição eletrônica dos elétrons de um átomo: hidrogênio, hélio e lítio
Science 8 and 9: Principles in writing electron configuration // (Tagalog-English Format)

3.5.4 - Composição do orbital atômico: Número quântico secundário (subcamada ou subnível de energia)

S1.3.3 / S1.3.4 Atomic orbitals and sub-levels
5.0 / 5 (0 votes)