Energy Levels and Spectra (Quantum Phenomena 5)
Summary
TLDRIn this video, the presenter explains energy levels in atoms, focusing on the concept of electron configuration and how electrons move between different energy levels. Using examples like the color spectrum and emission and absorption lines, the presenter shows how these transitions reveal unique characteristics of elements. The script further delves into calculations for determining wavelengths and frequencies related to photon emissions, particularly in hydrogen and mercury atoms. The video concludes with a discussion of hydrogen's energy levels and an introduction to wave-particle duality.
Takeaways
- 😀 Light passing through a prism creates a rainbow, which is a continuous spectrum of colors.
- 😀 The visible light spectrum ranges from 400 nm to 650 nm in wavelength.
- 😀 A glowing gas emits a discrete set of colors (emission lines) instead of the full spectrum.
- 😀 Each element emits a unique set of emission and absorption lines, which can be used to identify the element.
- 😀 The energy levels of an atom determine the wavelengths of light it absorbs or emits.
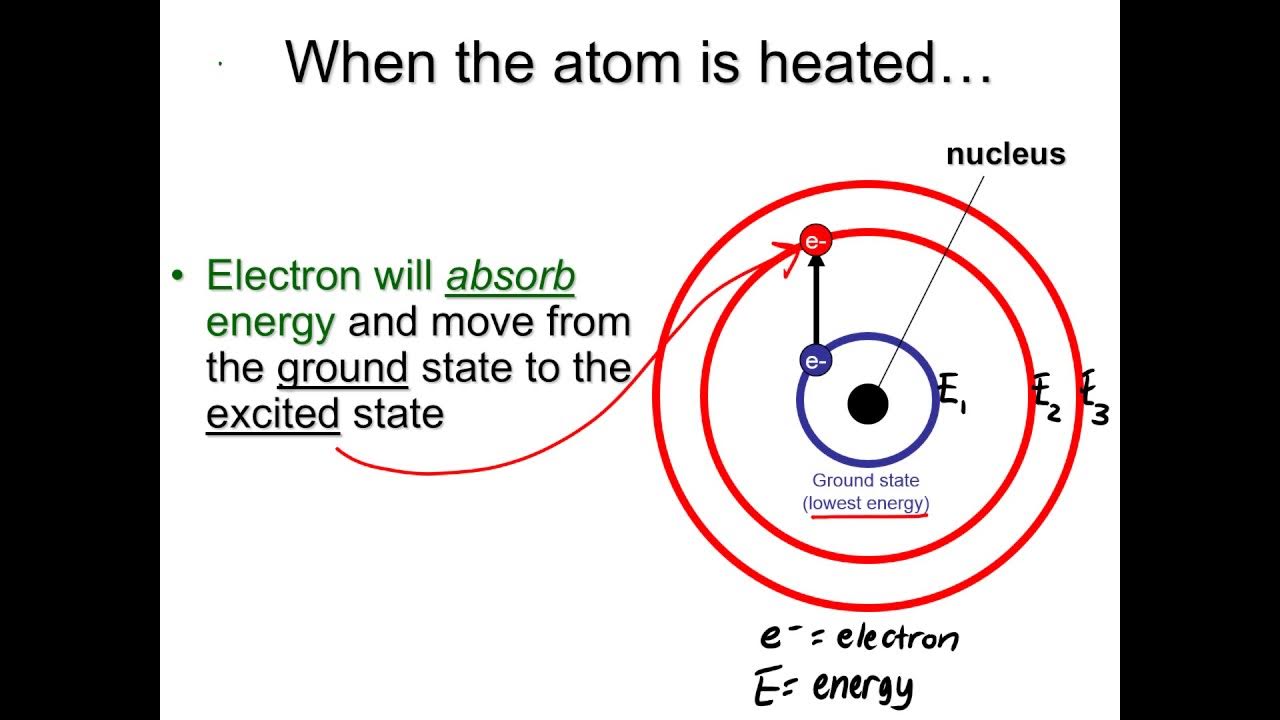
- 😀 Energy is absorbed when electrons move to higher energy levels (outer shells) and emitted when electrons drop to lower levels (inner shells).
- 😀 Emission lines are formed when electrons move to lower energy levels, while absorption lines occur when electrons move to higher levels.
- 😀 The wavelength of light emitted or absorbed is related to the energy change between the atom's energy levels.
- 😀 The energy of a photon is calculated by multiplying the frequency by Planck's constant.
- 😀 In the case of a mercury atom, the energy released when an electron drops from an excited state to the ground state is used to determine the wavelength of light.
- 😀 The hydrogen atom's energy levels follow a specific formula (E = -13.6 eV / n²) that determines the energy emitted when an electron transitions between states.
Q & A
What does the term 'energy levels' refer to in the context of atoms?
-Energy levels refer to the specific regions around an atom where electrons can exist. Electrons can move between these levels by either absorbing or emitting energy.
How does the color spectrum relate to the concept of energy levels in atoms?
-When light passes through a prism, it forms a continuous spectrum of colors. In contrast, when light is emitted by a gas containing specific elements, discrete emission lines appear, each corresponding to the energy differences between electron levels in the atoms of that gas.
What are 'emission lines' in a spectrum?
-Emission lines are discrete wavelengths of light emitted when electrons in an atom fall from a higher energy level to a lower one. Each element has a unique set of emission lines.
Why are the emission and absorption lines characteristic of each atom?
-The emission and absorption lines are characteristic because they correspond to specific energy transitions between electron levels unique to each atom. This makes it possible to identify the element producing the light based on its spectrum.
What is the difference between absorption and emission lines?
-Absorption lines are formed when electrons absorb energy to move to a higher energy level, while emission lines occur when electrons release energy as they drop to a lower energy level.
How can you calculate the energy of a photon released during an electron transition?
-The energy of the photon is calculated by the difference in energy between the initial and final energy levels of the electron. This energy can be converted to joules, and then the frequency and wavelength of the emitted light can be determined.
How do you calculate the frequency of a photon using energy?
-The frequency of a photon can be calculated using the formula: frequency = energy / Planck's constant. This relates the energy of the photon to its frequency.
How do you calculate the wavelength of a photon once you know its frequency?
-The wavelength of a photon can be calculated using the formula: wavelength = speed of light / frequency. By dividing the speed of light by the frequency, you get the wavelength in meters.
What role does the Planck's constant play in these calculations?
-Planck's constant is a fundamental constant used to relate the energy of a photon to its frequency. It allows us to calculate energy from frequency and vice versa.
What is the significance of the hydrogen atom’s energy levels?
-The energy levels of a hydrogen atom are quantized and represented by specific values. The formula used to calculate the energy at each level is E = -13.6 eV / n^2, where 'n' is the principal quantum number of the energy level.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Flame Tests Spectra Notes

Introduction to photoelectron spectroscopy | AP Chemistry | Khan Academy

Organização da eletrosfera [Módulo 02 - Aula 05]

Electrons and Energy Level, Electron Configuration | Grade 9 Science DepEd MELC Quarter 2 Module 1

Distribuição eletrônica [Módulo 02 - Aula 06]

Partikel Penyusun Benda dan Makhluk Hidup (Part-2) Konfigurasi Elektron, Ion dan Ikatan Ion
5.0 / 5 (0 votes)