1 Crystal Structure: 1.8 Structure of NaCl #SodiumChlorideStructure #NaCl #CrystalStructure
Summary
TLDRThis lecture explains the Face-Centered Cubic (FCC) diatomic structure of NaCl (sodium chloride) and its unit cell arrangement. It details the interpenetrating FCC sublattices of sodium and chlorine ions, the coordination number of 6, and the effective number of ions per unit cell. The transcript also covers the calculation of lattice constants, packing factor, and the positions of ions within the unit cell. Through diagrams and formulas, the video provides an in-depth understanding of NaCl's crystal structure and its properties, ideal for students studying material science or solid-state chemistry.
Takeaways
- 😀 NaCl is an example of an fcc diatomic crystal structure, which includes other materials like KBr, MgO, and MnO.
- 😀 In the NaCl structure, sodium ions (Na⁺) and chloride ions (Cl⁻) form two interpenetrating fcc monoatomic sublattices.
- 😀 The sodium ions and chloride ions alternate in the lattice, with each sublattice being monoatomic.
- 😀 The NaCl unit cell is cubic, with sodium and chloride ions occupying specific positions within the fcc lattice.
- 😀 Chlorine ions occupy all the fcc positions (corners and face centers) in the NaCl unit cell.
- 😀 Sodium ions occupy all the octahedral voids in the NaCl unit cell, which are at the body center and the midpoints of the edges.
- 😀 The two sublattices of sodium and chloride ions interpenetrate such that the origin of the chloride sublattice is at (0, 0, 0) and the sodium sublattice is at (a/2, 0, 0).
- 😀 The coordination number for both sodium and chloride ions in NaCl is 6, meaning each ion is surrounded by six opposite ions.
- 😀 The effective number of ions per unit cell in NaCl is 4 sodium ions and 4 chloride ions, contributing to 4 NaCl molecules per unit cell.
- 😀 The packing factor for the NaCl unit cell can be calculated using the formula involving the diameters of sodium and chloride ions, giving the fraction of the unit cell occupied by ions.
Q & A
What is the general structure of the NaCl crystal as described in the lecture?
-The NaCl crystal consists of two interpenetrating FCC (Face-Centered Cubic) monoatomic sublattices, one for sodium ions and one for chlorine ions. These sublattices form a single FCC diatomic lattice structure.
What are the key features of the FCC unit cell for chlorine ions in the NaCl structure?
-In the FCC unit cell for chlorine ions, there are eight corner atoms, each shared by eight unit cells, and six face-centered atoms, each shared by two unit cells. This results in a total of four chlorine ions per unit cell.
How are the sodium ions positioned in the NaCl unit cell?
-Sodium ions occupy the octahedral voids in the NaCl structure. These voids are located at the body center of the unit cell and at the midpoint of each edge. There are 12 edges in the unit cell, and each edge is shared by four unit cells.
How do the two sublattices of sodium and chlorine interpenetrate in the NaCl structure?
-The chlorine sublattice originates at (0, 0, 0), while the sodium sublattice originates at (a/2, 0, 0). This means that the two sublattices are interpenetrating, with each sodium ion positioned in the octahedral voids formed by the chlorine ions.
What is the coordination number of an ion in the NaCl structure, and how is it determined?
-The coordination number of each ion in the NaCl structure is 6. Each ion is surrounded by six oppositely charged ions. For example, each sodium ion is surrounded by six chlorine ions, and vice versa.
How is the lattice constant 'a' in NaCl related to the sizes of the sodium and chlorine ions?
-The lattice constant 'a' in NaCl is equal to the sum of the diameters of the sodium ion and the chlorine ion. The ions are in contact along the edge of the unit cell, so the edge length corresponds to the combined diameters of the two ions.
What is the packing factor of NaCl, and how is it calculated?
-The packing factor of NaCl is calculated as the ratio of the volume of the ions (sodium and chlorine) to the volume of the unit cell. The formula is: Packing factor = (4π/6) * [(diameter of sodium)^3 + (diameter of chlorine)^3] / (diameter of sodium + diameter of chlorine)^3.
What are octahedral voids in the context of the NaCl structure?
-Octahedral voids are spaces in the crystal lattice that are surrounded by eight faces. In the NaCl structure, sodium ions occupy these voids, which are located at the body center and at the midpoint of each edge of the unit cell.
What is the effective number of ions per unit cell in the NaCl structure?
-The effective number of ions per unit cell in NaCl is 4 sodium ions and 4 chlorine ions, making a total of 8 ions per unit cell. This corresponds to 4 NaCl molecules per unit cell.
How can the NaCl unit cell be drawn accurately for an exam?
-To draw the NaCl unit cell accurately, start by drawing a cube. Place chlorine ions at the eight corners and six face centers. Then, place sodium ions at the midpoint of each edge and at the body center. Ensure the ions are positioned correctly according to the FCC arrangement.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示
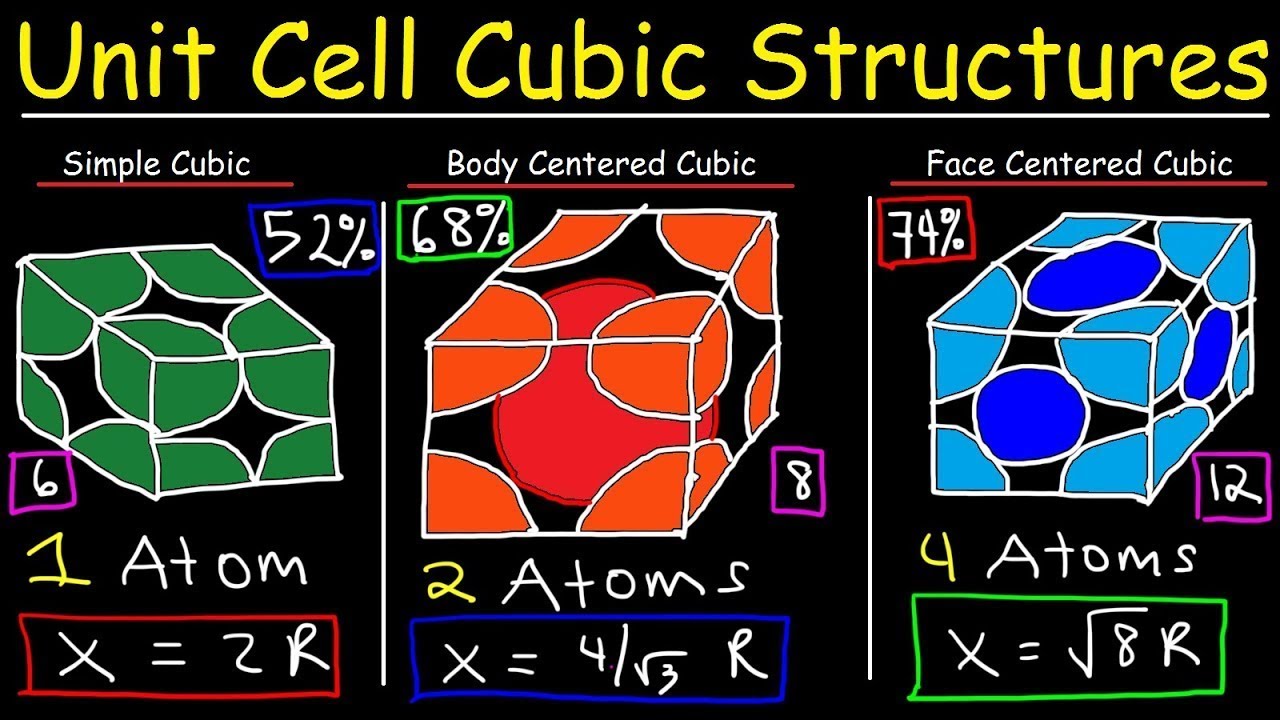
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu

Materiais - Módulo 6 - Estrutura dos materiais (Parte 6)
The primitive, body-centred and face-centred cubic unit cells

Lattice Structures Part 1

Aula 10 – Estruturas Cristalinas Cúbicas de Face Centrada, Corpo Centrado e Hexagonal Compacta.

AMIE Exam Lectures- Materials Science & Engineering | BCC | FCC | HCP | Cubic System | 3.2
5.0 / 5 (0 votes)
