Kuantum 2 .1 Hipotesis de Brogglie (Apakah Partikel Mempunyai sifat Gelombang?)
Summary
TLDRIn this lecture on quantum physics, the speaker reviews key phenomena that challenge classical physics, such as black body radiation, the photoelectric effect, and Compton scattering. The focus shifts to the dual nature of light, where light behaves both as a wave and a particle. The speaker introduces de Broglie's hypothesis that particles, like electrons, also exhibit wave-like properties. This hypothesis is explored through the relationship between momentum and wavelength, as de Broglie proposed that moving particles have an associated matter wave. The lecture sets the stage for further experimental confirmation of this wave-particle duality.
Takeaways
- 😀 The lecture continues from previous topics, focusing on quantum mechanics and the nature of light and particles.
- 😀 Classical physics failed to explain several phenomena, leading to the exploration of quantum mechanics.
- 😀 Key phenomena discussed include black body radiation, the photoelectric effect, and Compton scattering.
- 😀 Compton scattering revealed that when X-rays collide with electrons, there is a change in the wavelength, suggesting light behaves as particles.
- 😀 The dual nature of light is confirmed: light can behave both as a wave and as a particle, as demonstrated by Compton scattering and the photoelectric effect.
- 😀 There is a symmetry in physics: if waves have particle-like behavior, then particles should also exhibit wave-like behavior.
- 😀 The question arises: do particles have wave properties? This symmetry between particles and waves is a central hypothesis in quantum mechanics.
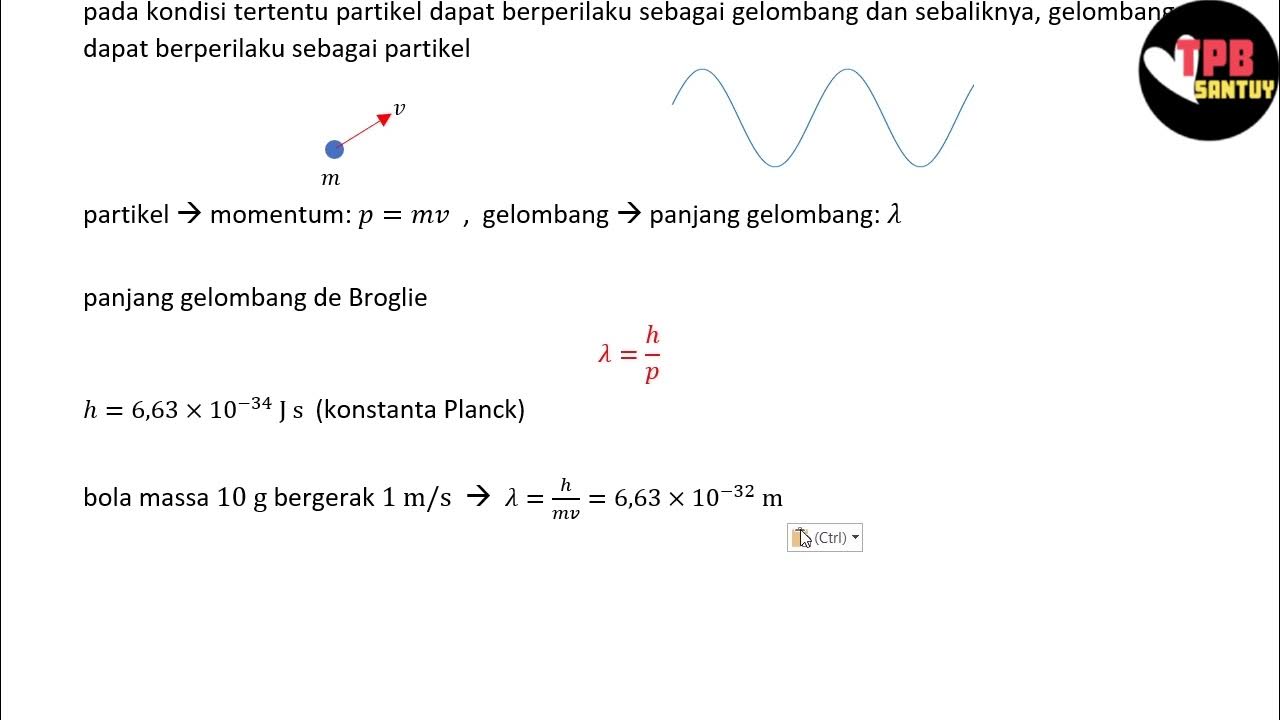
- 😀 De Broglie proposed the hypothesis that moving particles also have wave-like properties, specifically a matter wave.
- 😀 According to De Broglie, the wavelength of a moving particle is related to its momentum, expressed as λ = h / p, where 'h' is Planck's constant and 'p' is the momentum of the particle.
- 😀 De Broglie's hypothesis was a theoretical idea and required experimental validation. This idea was well-received and integrated into quantum mechanics.
- 😀 The mathematical form λ = h / p shows that particles with momentum also exhibit a corresponding wavelength, supporting the wave-particle duality in quantum mechanics.
Q & A
What is the main focus of this lecture?
-The lecture focuses on explaining quantum physics, particularly the wave-particle duality of light and particles, with reference to phenomena such as Compton scattering, the photoelectric effect, and the De Broglie hypothesis.
How does classical physics fail to explain certain phenomena?
-Classical physics fails to explain phenomena like black-body radiation, the photoelectric effect, and Compton scattering, which led to the development of quantum mechanics.
What was significant about the Compton scattering experiment?
-The Compton scattering experiment demonstrated that X-rays behave as particles (photons) when they scatter off stationary electrons, showing a shift in wavelength, which provided evidence for the particle-like nature of light.
How does light behave according to quantum theory?
-In quantum theory, light behaves both as a wave and as a particle (photon). This dual nature is confirmed through experiments such as the Compton scattering and the photoelectric effect.
What is the wave-particle duality?
-Wave-particle duality is the concept that particles such as light and electrons exhibit both wave-like and particle-like properties. This was shown experimentally for light and later extended to matter particles like electrons.
Why did scientists hypothesize that particles might exhibit wave-like properties?
-The idea that particles might exhibit wave-like properties arose from the symmetry in quantum physics. If waves can behave like particles (as in the photoelectric effect), then it was hypothesized that particles might also show wave-like behavior.
What was De Broglie’s contribution to quantum theory?
-De Broglie proposed that particles, like electrons, have associated wave-like properties. He hypothesized that the wavelength of a moving particle is inversely proportional to its momentum, described by the formula λ = h / p.
How does the equation λ = h / p relate to the De Broglie hypothesis?
-The equation λ = h / p expresses the wavelength (λ) of a particle in terms of its momentum (p), with h being Planck’s constant. This relationship shows that particles can have wave-like characteristics depending on their momentum.
What is the difference between a hypothesis and a postulate in quantum physics?
-A hypothesis in quantum physics is a theoretical proposition based on reasoning or symmetry, without experimental proof. A postulate, however, is a fundamental assumption made to explain experimental results that cannot be explained by existing theories.
How was De Broglie’s hypothesis tested and accepted?
-De Broglie’s hypothesis was initially theoretical. However, it gained support as experiments, such as electron diffraction, confirmed the wave-like behavior of particles, validating the idea that particles exhibit both particle and wave characteristics.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados

AKTU - QUANTUM MECHANICS for Engineering Physics
Dualisme Gelombang-Partikel | Fenomena Kuantum | Part 1 | Fisika Dasar

KONSEP DAN FENOMENA KUANTUM - MATERI FISIKA KELAS XII
What is Compton Scattering?

3.3.1 - Radiação eletromagnética: Teoria Quântica - Emissão de luz por objetos quentes (Planck)
Gamma Rays
5.0 / 5 (0 votes)
