Deviations from Beer-Lambert law
Summary
TLDRThis video explores deviations from Beer-Lambert Law, highlighting its significance in analytical chemistry. It explains how absorbance is expected to correlate linearly with analyte concentration, but deviations can occur due to real, spectral, and chemical factors. Real deviations arise at high concentrations due to refractive index changes, while spectral deviations occur with polychromatic radiation, which can be mitigated by using monochromatic light. Chemical deviations result from changes in the analyte's state, like pH variations. Understanding these deviations is crucial for accurate spectrophotometric measurements, enhancing analytical accuracy in laboratory settings.
Takeaways
- 😀 The Beer-Lambert Law relates absorbance (A) to analyte concentration (C) through the formula A = abc, where 'a' is absorptivity.
- 😀 Absorbance is directly proportional to concentration, resulting in a straight line through the origin when plotted.
- 😀 Deviations from Beer-Lambert Law can lead to non-linear absorption curves, indicating potential errors in measurement.
- 😀 There are three types of deviations from Beer-Lambert Law: real deviation, spectral deviation, and chemical deviation.
- 😀 Real deviation occurs at high concentrations of analytes, affecting the speed of light and refractive index, leading to inaccuracies in absorbance measurement.
- 😀 To minimize real deviation, samples should be prepared in low concentrations (10^-6 to 10^-3 M).
- 😀 Spectral deviation is caused by using polychromatic radiation, which can result in other substances absorbing light, affecting measurement accuracy.
- 😀 Using a monochromator can help eliminate spectral deviation by ensuring the use of monochromatic radiation with a narrow bandwidth.
- 😀 Chemical deviation results from changes in association, dissociation, or pH of the sample, affecting absorptivity and leading to deviations.
- 😀 To prevent chemical deviation, it is essential to monitor sample conditions and maintain a stable pH using suitable buffer systems.
Q & A
What does the Beer-Lambert law state?
-The Beer-Lambert law states that absorbance (A) is directly proportional to the concentration (c) of the analyte in a solution, expressed as A = abc, where 'a' is the absorptivity.
What is meant by deviations from the Beer-Lambert law?
-Deviations from the Beer-Lambert law refer to instances where the relationship between absorbance and concentration does not follow the expected linear pattern, leading to non-linear absorption curves.
What are the three types of deviations mentioned in the video?
-The three types of deviations from the Beer-Lambert law are real deviations, spectral deviations, and chemical deviations.
What causes real deviations?
-Real deviations occur primarily at high concentrations of analytes, where the refractive index of the solution affects the speed of light and consequently the absorbance measurement.
How can real deviations be minimized?
-Real deviations can be minimized by preparing samples at very low concentrations, specifically between 10^-6 to 10^-3 molar, to reduce the effects of refractive index.
What is the difference between monochromatic and polychromatic radiation?
-Monochromatic radiation has a narrow bandwidth (e.g., 550-570 nm), while polychromatic radiation has a wider bandwidth (e.g., 400-700 nm), which can lead to spectral deviations when analyzing absorbance.
What is stray radiation, and how does it affect measurements?
-Stray radiation is unwanted light that reaches the detector due to reflections, refractions, or scattering. It can lead to inaccurate absorbance measurements and spectral deviations.
How can spectral deviations be prevented?
-Spectral deviations can be prevented by using monochromatic radiation and employing a suitable monochromator that narrows the wavelength range of the radiation sent through the sample.
What is a chemical deviation?
-Chemical deviation is observed when there are changes in the sample's composition, such as association or dissociation of molecules or changes in pH, which affect absorptivity.
How can chemical deviations be minimized?
-Chemical deviations can be minimized by ensuring that the sample's pH is constant, often achieved through the use of buffer systems, and by monitoring any possible changes in molecular associations.
Outlines

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraMindmap

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraKeywords

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraHighlights

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraTranscripts

Esta sección está disponible solo para usuarios con suscripción. Por favor, mejora tu plan para acceder a esta parte.
Mejorar ahoraVer Más Videos Relacionados
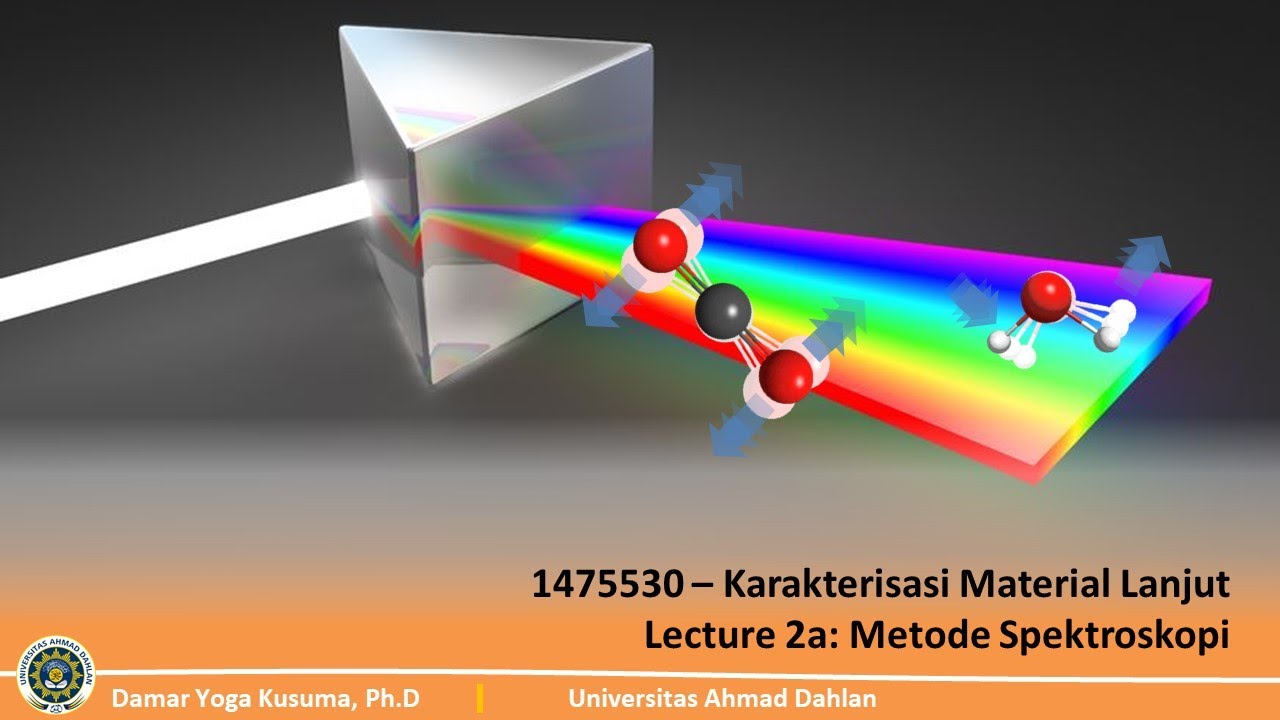
UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)

Espectrometria de absorção molecular - Parte 1: Fundamentos
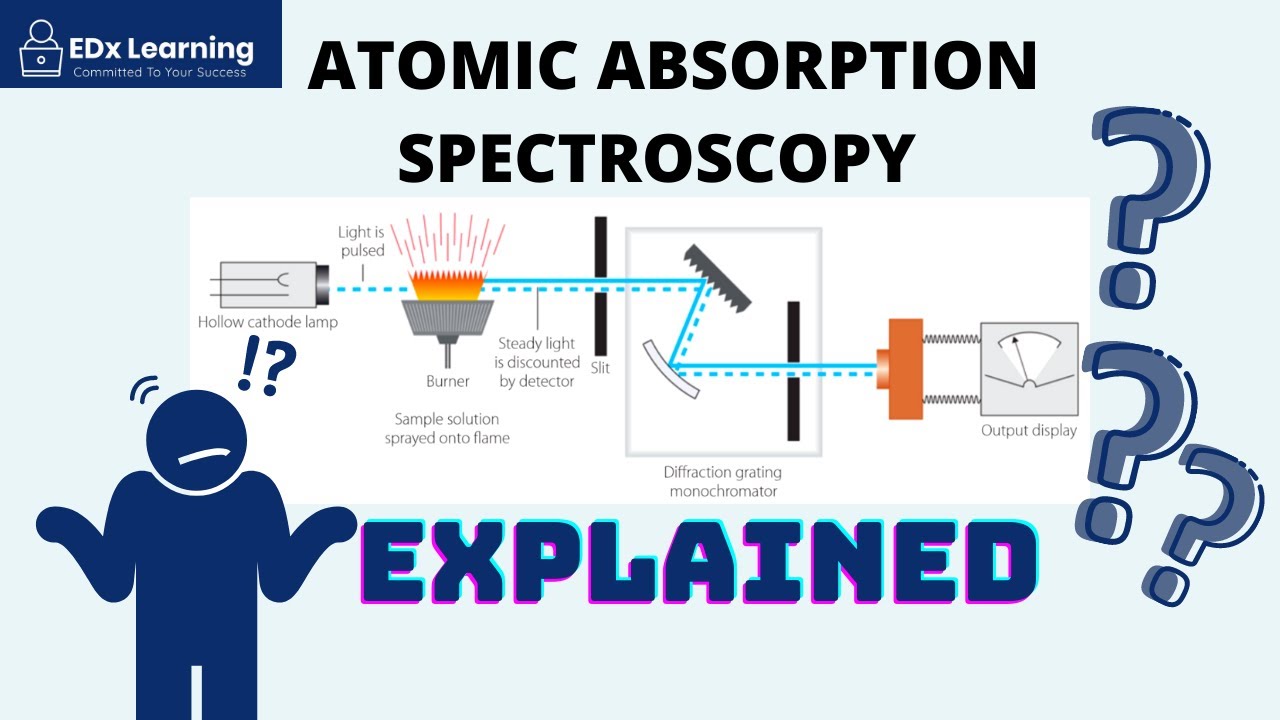
Atomic Absorption Spectroscopy (AAS) Explained - PART 1
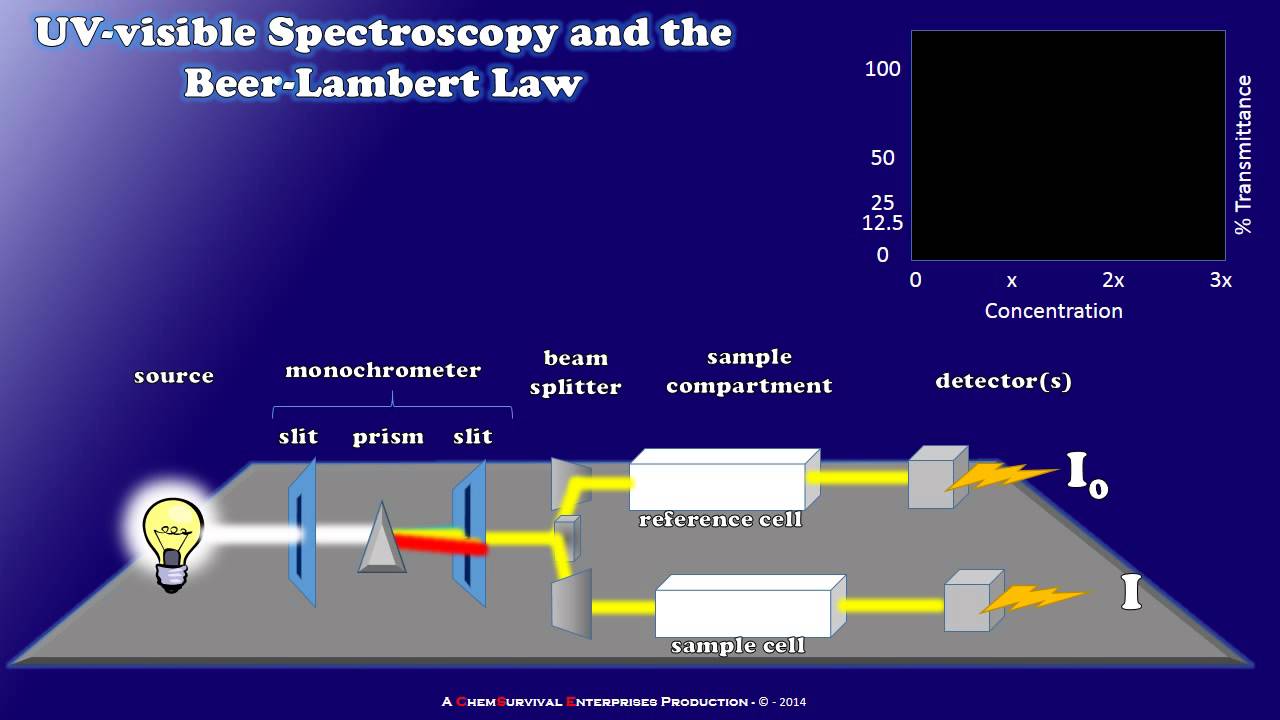
How a Simple UV-visible Spectrophotometer Works

UV Vis spectroscopy explained lecture
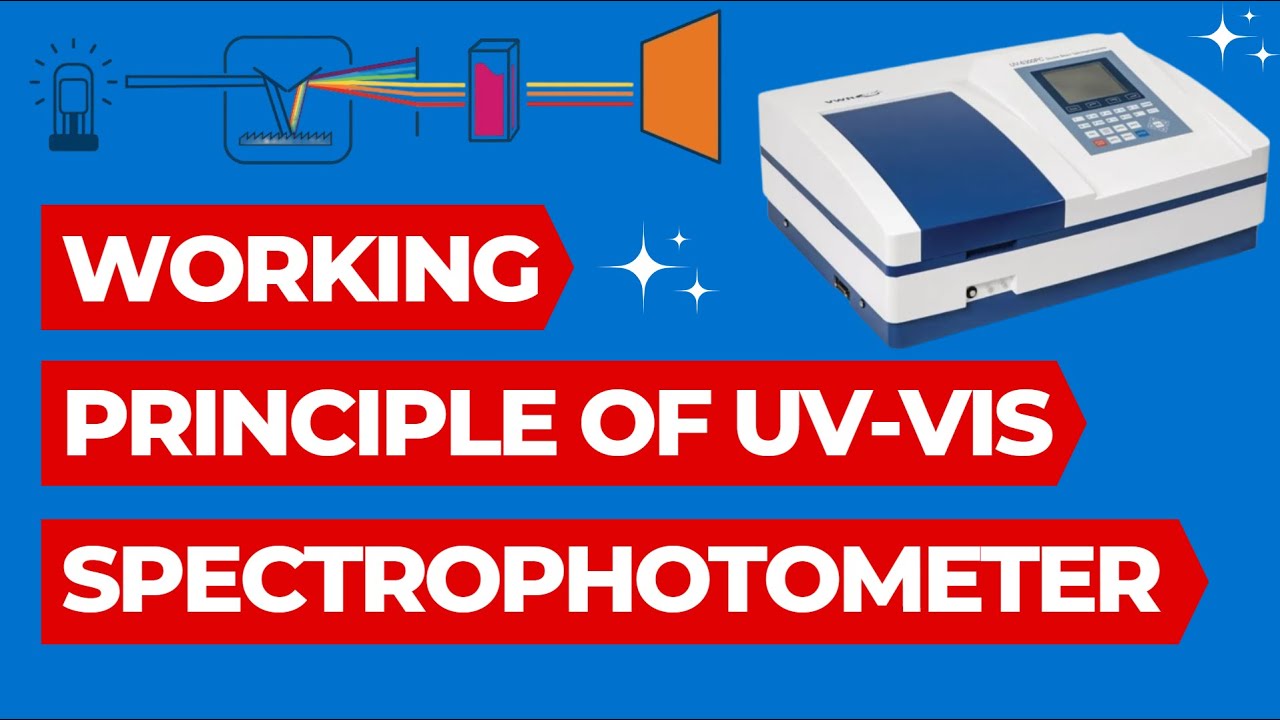
Working Principle of UV Spectrophotometer
5.0 / 5 (0 votes)
