How a Simple UV-visible Spectrophotometer Works
Summary
TLDRIn this video, Professor Davis from Chem Survival introduces the basics of UV-visible spectroscopy and the Beer-Lambert Law. He walks viewers through the components of a typical spectrophotometer, explaining the role of each part, such as the light source, monochromator, beam splitter, and detectors. The professor demonstrates how light interacts with a sample, showing how absorbance and transmittance are related. He emphasizes the importance of converting transmittance data to absorbance using the Beer-Lambert Law to create a linear relationship, making data interpretation easier for scientific analysis. Overall, the video provides a clear and practical introduction to spectroscopy concepts.
Takeaways
- 😀 The script introduces UV-visible spectroscopy and the Beer-Lambert Law, explaining the basic components of a spectrophotometer.
- 🔬 A typical UV-visible spectrophotometer includes a source lamp, monochromator, beam splitter, sample compartment, and detectors.
- 💡 The light source can range from a simple scooter headlamp to more advanced lamps like deuterium or xenon arc lamps.
- 🔄 The monochromator separates light into different wavelengths using slits and a prism or diffraction grating.
- 🔑 The beam splitter divides the light into two equal, parallel beams which travel through separate reference and sample cells.
- 📉 The detectors convert the light intensity into electrical current, which is then monitored by a computer.
- 🧪 When a sample is added to the sample cell, it absorbs light, causing a decrease in light intensity exiting the cell.
- 📊 The relationship between concentration and percent transmittance is exponential, not linear, in a typical spectrophotometric setup.
- 🔍 To simplify data analysis, the Beer-Lambert Law converts percent transmittance into absorbance, making the relationship linear.
- 🔢 Using absorbance rather than transmittance allows for easier interpolation or extrapolation within data sets, aiding in more accurate predictions.
Q & A
What is the purpose of the monochromator in a UV-Visible spectrophotometer?
-The monochromator is used to separate light into individual wavelengths. It achieves this using slits and a prism or diffraction grating, allowing only one specific wavelength of light to pass through at a time.
Why is the light split into two beams in the spectrophotometer setup?
-The light is split into two beams by a beam splitter. One beam passes through the sample, while the other passes through the reference. This allows for comparison of the light intensity after interacting with the sample and the reference to measure the absorbance.
How does the intensity of light change when a sample is added to the sample cell?
-When a sample is added, it absorbs some of the light, causing the intensity of light exiting the sample cell to decrease. As a result, the current generated by the detector also decreases.
What happens to the light intensity as the concentration of the sample increases?
-As the concentration of the sample increases, more light is absorbed, causing the intensity of light exiting the sample cell to decrease further. This leads to a reduction in the detected current, which is correlated to the concentration of the sample.
Why is the relationship between concentration and transmittance exponential rather than linear?
-The relationship is exponential because the absorption of light by the sample follows a non-linear pattern. As the concentration increases, each additional increment of the sample absorbs a fixed percentage of light, leading to a progressively larger reduction in transmittance.
What is the Beer-Lambert Law and how does it help in spectrophotometric analysis?
-The Beer-Lambert Law is a mathematical relationship that describes how absorbance is related to concentration. It allows scientists to convert the non-linear, exponential relationship between concentration and transmittance into a linear relationship between concentration and absorbance, making data analysis easier.
Why is it useful to convert transmittance into absorbance in UV-Visible spectroscopy?
-Converting transmittance into absorbance makes the data linear and easier to interpret. This simplification allows for more straightforward predictions and data analysis, especially when correlating absorbance to concentration.
What is the significance of the negative logarithm in the Beer-Lambert Law?
-The negative logarithm of transmittance is used to convert the exponential relationship between transmittance and concentration into a linear relationship, which makes the data easier to analyze and interpret.
How do detectors work in a spectrophotometer?
-Detectors in a spectrophotometer convert the impact of photons (light) into electrical current. The amount of current generated is proportional to the intensity of light detected, which can be measured by a computer.
What would happen if both the sample and reference cells showed identical intensities of light?
-If both cells showed identical intensities, it would indicate that the sample does not absorb any light, meaning its concentration is zero, or the sample does not interact with the specific wavelengths of light being analyzed.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

🧪 Spectroscopie d'absorption UV-visible (avec @myMaxicours)

UV Vis spectroscopy explained lecture
UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)

SPEKTROFOTOMETRI UV-Vis | Prinsip Kerja Spektrofotometri UV-Vis
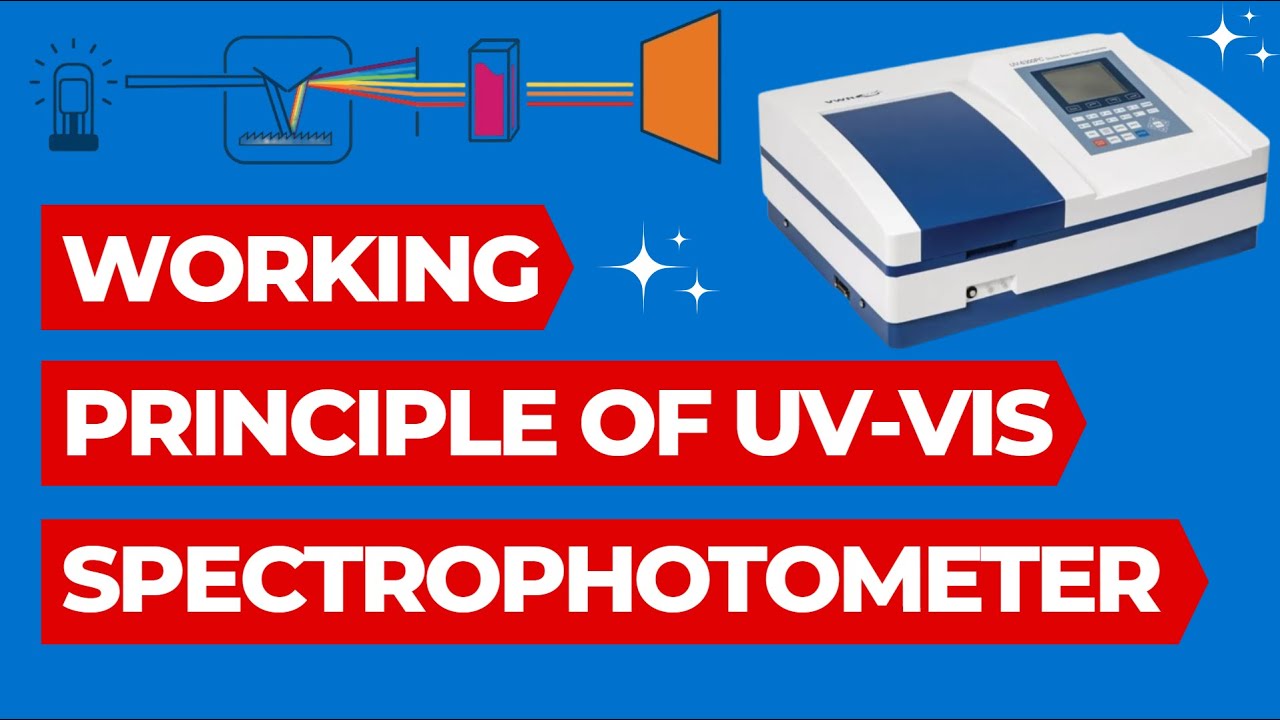
Working Principle of UV Spectrophotometer

A short history of the periodic table
5.0 / 5 (0 votes)