UV Vis spectroscopy explained lecture
Summary
TLDRThis video tutorial from 'Somos Biology' delves into the fundamentals of UV-Visible spectroscopy, a technique widely used in chemistry and life sciences for determining the concentration of molecules in solutions. The script explains the setup of a spectrophotometer, the molecular mechanism behind light absorption, and how this technique can provide insights into chemical groups present in molecules. It also discusses the importance of 'lambda max' for identifying maximum absorbance and the use of the Beer-Lambert law for calculating unknown concentrations, making complex scientific concepts accessible to viewers.
Takeaways
- 🌟 UV-Visible spectroscopy is a widely used technique in both chemistry and life sciences for various experiments and analyses.
- 🔍 It is primarily used for determining the concentration of different substances or molecules in a solution, including cells in microbiology practicals.
- 📈 The technique can also provide some information about the chemical groups present in molecules, though the data is not highly reliable for differentiating between different types of groups.
- 🛠️ The setup of a UV-Visible spectrophotometer includes a light source, a monochromator to separate light wavelengths, a beam separator, and a detector to measure light intensity.
- 🌈 The UV-Visible range of the electromagnetic spectrum is from 200 to 800 nanometers, with UV ranging from 200 to 400 nm and visible light from 400 to 800 nm.
- 🔬 The molecular mechanism involves the absorption of light energy by molecules, causing electrons to jump from a ground state to a higher energy state, which is specific to certain wavelengths.
- 📊 Beer-Lambert Law is used to relate the absorbance of light to the concentration of a molecule in a solution, which is crucial for quantitative analysis.
- 📉 A plot of absorbance against concentration typically results in a non-linear curve, which can be linearized by using a logarithmic scale to facilitate easier calculation of unknown concentrations.
- 📚 Understanding the specific wavelength (lambda max) at which a molecule absorbs light most strongly is important for identifying the maximum absorbance for different chemical groups.
- 🔬 The molecular level process of UV-Visible spectroscopy involves the excitation of electrons from bonding or non-bonding orbitals to higher energy states, such as sigma or pi orbitals.
- 👍 The video concludes by emphasizing the importance of UV-Visible spectroscopy for concentration analysis and its limitations in identifying specific chemical bonds.
Q & A
What is UV-visible spectroscopy and why is it used?
-UV-visible spectroscopy is a technique widely used in chemical and life sciences to measure the concentration of different substances in a solution. It is also used to identify chemical groups present in molecules, although the data is not highly reliable for differentiating between different groups.
What are the main applications of UV-visible spectroscopy?
-UV-visible spectroscopy is used for determining the concentration of molecules in a solution, including in microbiology for measuring cell concentrations. It is also used in enzymatic reactions and enzyme kinetic studies to assess enzyme-associated activities.
How does the UV-visible spectrophotometry work?
-UV-visible spectrophotometry works by passing light through a sample and measuring the intensity of the light that is transmitted through it. The difference in intensity before and after the sample is used to calculate the absorbance, which is related to the concentration of the molecules in the sample.
What is the role of a monochromator in UV-visible spectroscopy?
-A monochromator is a device that separates the light from a source into its different wavelengths. It allows only a specific wavelength of light to pass through, which is then used to analyze the sample.
What is the significance of the wavelength in UV-visible spectroscopy?
-The wavelength is significant because different molecules absorb different wavelengths of light. By knowing the specific wavelength that a molecule absorbs, one can identify the presence of certain chemical groups within the molecule.
What is the relationship between frequency, wavelength, and energy of an electromagnetic wave?
-The frequency and energy of an electromagnetic wave are directly proportional; higher frequency means higher energy. The wavelength and frequency are inversely proportional; shorter wavelength means higher frequency and vice versa.
How is the concentration of a molecule in a solution determined using UV-visible spectroscopy?
-The concentration is determined by measuring the absorbance of the sample at a specific wavelength. Using the Beer-Lambert Law, which states that absorbance is proportional to the concentration of the molecule, the concentration can be calculated.
What is meant by 'transmittance' in the context of UV-visible spectroscopy?
-Transmittance is the ratio of the intensity of light after passing through the sample (I) to the initial intensity of light (I0). It indicates the amount of light that has been transmitted through the sample.
What is the difference between transmittance and absorbance?
-Transmittance measures the amount of light that passes through the sample, while absorbance measures the amount of light that is absorbed by the sample. They are inversely related; higher transmittance means lower absorbance and vice versa.
Why is it difficult to extrapolate data from a graph of percent transmittance versus concentration?
-It is difficult because the relationship between percent transmittance and concentration is not linear, making it challenging to predict the concentration of unknown samples from such a graph.
How can a graph of absorbance versus concentration be made linear for easier data interpretation?
-By plotting the logarithm of the concentration (or the reciprocal of transmittance) against absorbance, the graph becomes linear, which simplifies the process of extrapolating the concentration of unknown samples.
What is the molecular mechanism behind the absorption of light in UV-visible spectroscopy?
-The molecular mechanism involves the excitation of electrons within molecules from a lower energy state (ground state) to a higher energy state when they absorb energy from light of a specific wavelength. The energy absorbed corresponds to the difference in energy levels between the molecular orbitals.
Why are UV-visible spectra not reliable for identifying specific chemical bonds?
-UV-visible spectra are not reliable for identifying specific chemical bonds because many molecules with similar bonds and structures absorb light at similar wavelengths, making it difficult to distinguish between them based on UV-visible spectroscopy alone.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)

Ultraviolet/Visible Spectroscopy (UV-Vis)

Chemistry Class 12 | Chapter 12 | Topic 3b | UV-VIS Spectroscopy | in urdu | tutoria.pk
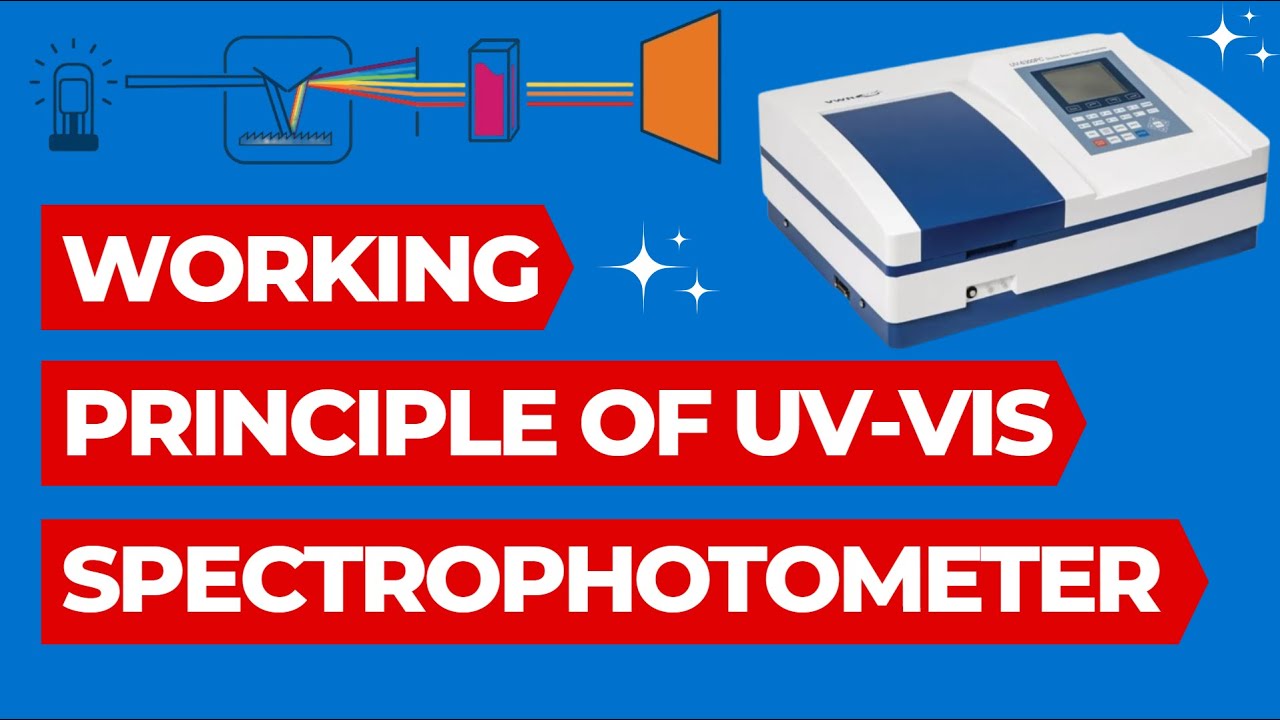
Working Principle of UV Spectrophotometer

Introduction to Biology: What is Life?

Spektroskopi Raman
5.0 / 5 (0 votes)