Atomic Absorption Spectroscopy (AAS) Explained - PART 1
Summary
TLDRThis chemistry lesson explores Atomic Absorption Spectroscopy (AAS), a technique for determining metal ion concentrations. It explains the interaction of matter with electromagnetic radiation, emphasizing how elements selectively absorb specific wavelengths. The process involves atomizing a metal cation solution in a flame, exposing it to radiation, and measuring the absorbance to calculate concentration using the Beer-Lambert law. The lesson also covers the complementary nature of emission and absorption spectra, crucial for understanding AAS.
Takeaways
- 🔬 AAS (Atomic Absorption Spectroscopy) is used to analyze ions, especially metal ions in a solution, and is essential for both explaining its methodology and solving concentration calculation problems.
- 🌈 Spectroscopy studies how matter interacts with energy, specifically electromagnetic radiation (EMR), which travels at the speed of light and is categorized by frequency and wavelength.
- ⚡ High energy waves have high frequency and short wavelength, while low energy waves have low frequency and long wavelength, and they are inversely related as per the equation c = fλ (speed of light = frequency × wavelength).
- 🧐 Different elements are selective about the EMR they absorb and will only take in specific wavelengths, which can be used to analyze their concentration in solutions.
- 📈 The degree of absorbance of specific wavelengths is directly proportional to the concentration of an element in a solution, as shown by the modified Beer-Lambert equation A = kC (absorbance = constant × concentration).
- 🔋 When elements absorb EMR, their electrons jump to higher energy levels, and when they return to lower levels (ground state), they re-emit the absorbed energy as EMR.
- 🎨 The absorption spectrum of an element is complementary to its emission spectrum, meaning the wavelengths absorbed are the same as those re-emitted.
- 🧪 AAS is particularly useful for measuring trace amounts of metal cations in a solution, even in very small concentrations such as parts per million or billion.
- 🔥 The sample solution is atomized in a flame or furnace, and metal atoms absorb specific wavelengths of light, allowing their absorbance to be measured and their concentration calculated.
- 📊 By measuring absorbance and using a calibration curve, the concentration of metal ions in a solution can be accurately determined using AAS.
Q & A
What is the main purpose of Atomic Absorption Spectroscopy (AAS) as discussed in the video?
-The main purpose of AAS is to determine the concentration of metal ions in a solution by measuring the absorbance of specific wavelengths of electromagnetic radiation.
What are the two key points to know about AAS for exams?
-First, you must be able to explain the methodology of AAS. Second, you should be prepared to perform calculation questions to determine the concentration of metal ions in a solution using AAS.
How does spectroscopy help in determining the concentration of elements in a sample?
-Spectroscopy studies how matter interacts with energy. By introducing electromagnetic radiation and observing how a substance absorbs specific wavelengths, you can determine the concentration of particular elements in the sample.
What type of energy is used in AAS, and how is it categorized?
-AAS uses electromagnetic radiation, which travels at the speed of light (approximately 3 x 10^8 meters per second). It is categorized based on its frequency and wavelength, where high-energy waves have high frequencies and low wavelengths, and low-energy waves have low frequencies and longer wavelengths.
What is the relationship between absorbance and concentration in AAS?
-The absorbance of specific wavelengths is directly proportional to the concentration of the element in the sample. As absorbance increases, the concentration also increases, which is summarized by the equation A = kC, where A is absorbance, k is a constant, and C is concentration.
How do elements absorb electromagnetic radiation in AAS?
-Elements are selective in absorbing certain wavelengths of electromagnetic radiation. When exposed to the full electromagnetic spectrum, they only absorb specific wavelengths that correspond to the energy needed to excite their electrons to higher energy levels.
What happens to an electron in an atom when it absorbs electromagnetic radiation?
-When an electron absorbs electromagnetic radiation, it gains energy and moves to a higher energy level. Eventually, it de-excites back to its ground state, re-emitting the same amount of energy in the form of electromagnetic radiation.
What is the significance of an element's emission and absorption spectra in AAS?
-An element's emission and absorption spectra are complementary. The wavelengths of light absorbed by the element are the same as those it emits when its electrons return to their ground state. AAS focuses on the absorption spectra to analyze the concentration of elements.
Can AAS measure trace amounts of metal ions in a solution? If so, how accurately?
-Yes, AAS can measure trace amounts of metal ions, even at concentrations as low as parts per million (ppm) or parts per billion (ppb), making it a highly accurate technique for quantitative ion analysis.
What is the role of the monochromator in AAS?
-The monochromator isolates a specific wavelength of light that corresponds to the absorption spectra of the element being studied. This allows for more precise analysis of the absorbance, which is used to determine the concentration of the element.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Metode pengujian logam Fe dengan AAS

Determination of Zinc by Linear Calibration and Standard Addition Methods using AAS
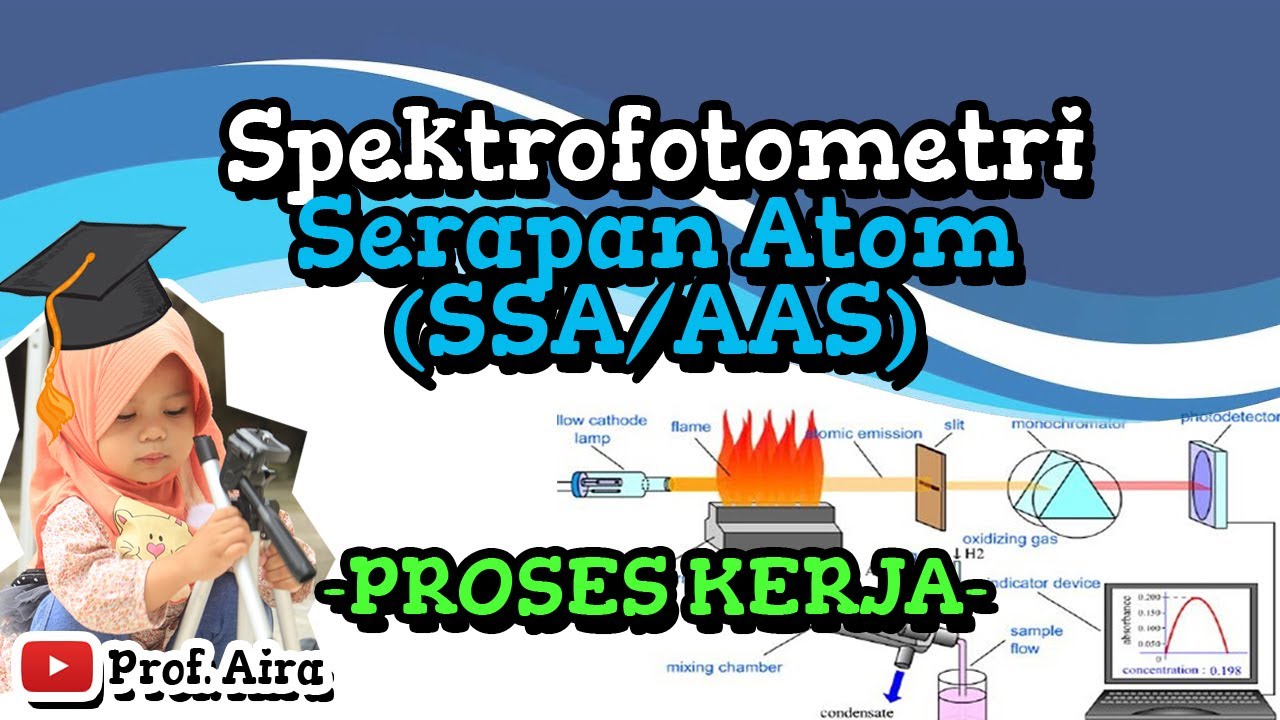
SPEKTROFOTOMETRI SERAPAN ATON (SSA/AAS) – PROSES KERJA

UV Vis spectroscopy explained lecture

Quickly Understand Atomic Absorption Spectroscopy (AAS)
UAD - Kuliah Online 1475530 Karakterisasi Material Lanjut (Lecture 2a - part 1)
5.0 / 5 (0 votes)