Molality and Colligative Properties
Summary
TLDRIn this educational video, Professor Dave explains colligative properties, which describe how solute particles in a solution affect physical processes like phase changes. He introduces molality as a measure of solute concentration and discusses how it impacts vapor pressure lowering, boiling point elevation, and freezing point depression. The video covers the interference of solute with solvent activity at phase interfaces and the use of constants (Kb and Kf) to calculate these effects. It concludes with a real-world example of using salt to lower the freezing point of water on icy streets.
Takeaways
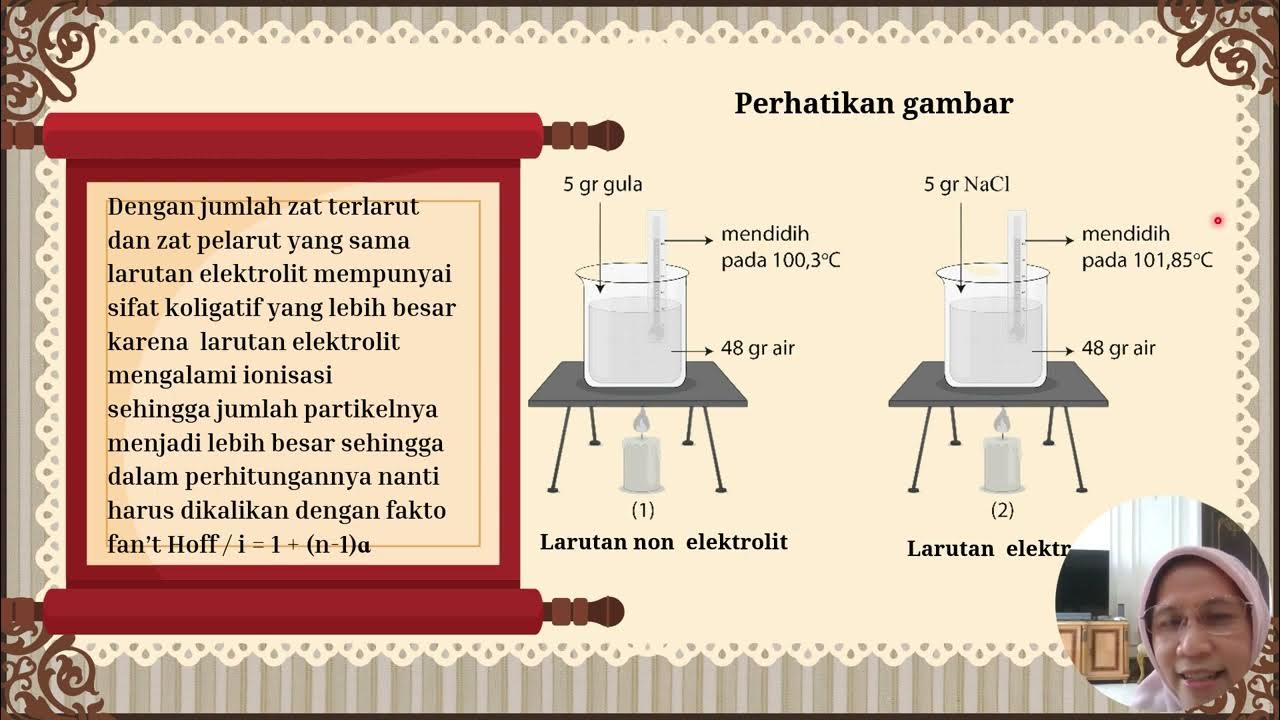
- 🔬 Colligative properties are physical properties of a solution that depend on the concentration of solute particles, not their chemical identity.
- 🌡️ Adding solute to a solvent affects vapor pressure, boiling point, and freezing point due to the interference of solute particles with the solvent's physical processes.
- 🧪 Molality, expressed as moles of solute per kilogram of solvent, is a preferred measure of concentration for discussing colligative properties.
- 💧 Solute particles at the liquid's surface reduce vapor pressure by occupying surface area and hindering solvent molecules from evaporating.
- 🌡️ Boiling point elevation occurs because solute particles block solvent molecules from vaporizing, requiring additional heat energy.
- ❄️ Freezing point depression happens as solute particles interfere with the solvent's ability to form a crystalline lattice, necessitating a lower temperature for freezing.
- 📚 Constants Kb and Kf, specific to a solvent, relate molality to the changes in boiling and freezing points, respectively.
- ➕ The change in boiling point is added to the original boiling point, while the change in freezing point is subtracted from the original freezing point.
- ⛄️ Salt is added to icy streets to lower the freezing point of water, keeping it liquid and reducing ice formation.
- 📧 The tutorial encourages viewers to subscribe for more content and to reach out with questions via email.
Q & A
What are colligative properties?
-Colligative properties are physical properties of a solution that depend only on the concentration of solute particles and not on their chemical identity. They include vapor pressure lowering, boiling point elevation, and freezing point depression.
How does the presence of solute particles affect the vapor pressure of a solution?
-Solute particles at the surface of the liquid occupy some of the surface area, hindering solvent molecules from evaporating. This causes the vapor pressure of the liquid to decrease.
What is molality and how is it different from molarity?
-Molality is the number of moles of solute per kilogram of solvent, whereas molarity is the number of moles of solute per liter of solution. Molality is often used when discussing colligative properties because it is independent of the volume of the solution.
How is the vapor pressure of a solution related to the mole fraction of the solvent?
-The new vapor pressure of a solution is equal to the vapor pressure of the pure solvent multiplied by the mole fraction of the solvent, which is the percentage of particles in the solution that are solvent molecules.
Why does the boiling point of a solution increase when solute is added?
-Solute particles block solvent molecules from entering the gas phase, requiring more heat energy to achieve boiling. This results in an elevated boiling point for the solution.
What is the relationship between the change in boiling point and the molality of a solution?
-The change in boiling point is given by the molality of the solution times a constant specific to the solvent, known as Kb.
How does the presence of solute affect the freezing point of a solution?
-Solute particles interfere with the ability of solvent particles to form a lattice structure, which is necessary for freezing. This requires the system to reach a lower temperature to freeze, resulting in freezing point depression.
What is the formula for calculating the change in freezing point in a solution?
-The change in freezing point is given by the molality of the solution times another constant specific to the solvent, known as Kf.
Why is salt added to icy streets in winter?
-Salt is added to icy streets to lower the freezing point of water, causing it to remain liquid at lower temperatures and thus reducing the formation of ice.
How can one find the Kb and Kf constants for different solvents?
-The Kb and Kf constants can be found in tables in textbooks or online resources and are specific to each solvent.
What is the significance of adding or subtracting the change in boiling and freezing points from their original values?
-The change in boiling point is added to the original boiling point because solute always raises it, while the change in freezing point is subtracted from the original freezing point because solute always lowers it.
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführen5.0 / 5 (0 votes)