SIFAT KOLIGATIF LARUTAN - KIMIA - MATERI UTBK SBMPTN DAN SIMAK UI
Summary
TLDRIn this educational video, Kak Tia from Epson introduces the concept of colligative properties of solutions to Grade 12 students. She explains that colligative properties, including changes in vapor pressure, boiling point, freezing point, and osmotic pressure, are influenced only by the amount of solute in a solution, not the type of solute. The video also covers the Van’t Hoff factor, distinguishing between strong and weak electrolytes and non-electrolytes. Additionally, it provides insights into solution concentrations like molarity and molality, making complex concepts accessible for learners and encouraging further study of chemistry.
Takeaways
- 😀 Colligative properties of solutions depend only on the amount of solute, not the type of solute or solvent.
- 😀 There are four main colligative properties: decrease in vapor pressure, increase in boiling point, freezing point depression, and osmotic pressure.
- 😀 The pH of a solution is not a colligative property because it depends on the type of substance, unlike colligative properties that depend on the amount of solute.
- 😀 To determine the color properties of a substance, you need to know its type, which means color is not a colligative property either.
- 😀 A solution consists of two components: the solute (the substance being dissolved) and the solvent (the substance doing the dissolving).
- 😀 The amount of solute in a solution can be measured using different quantities such as mass, concentration, and volume.
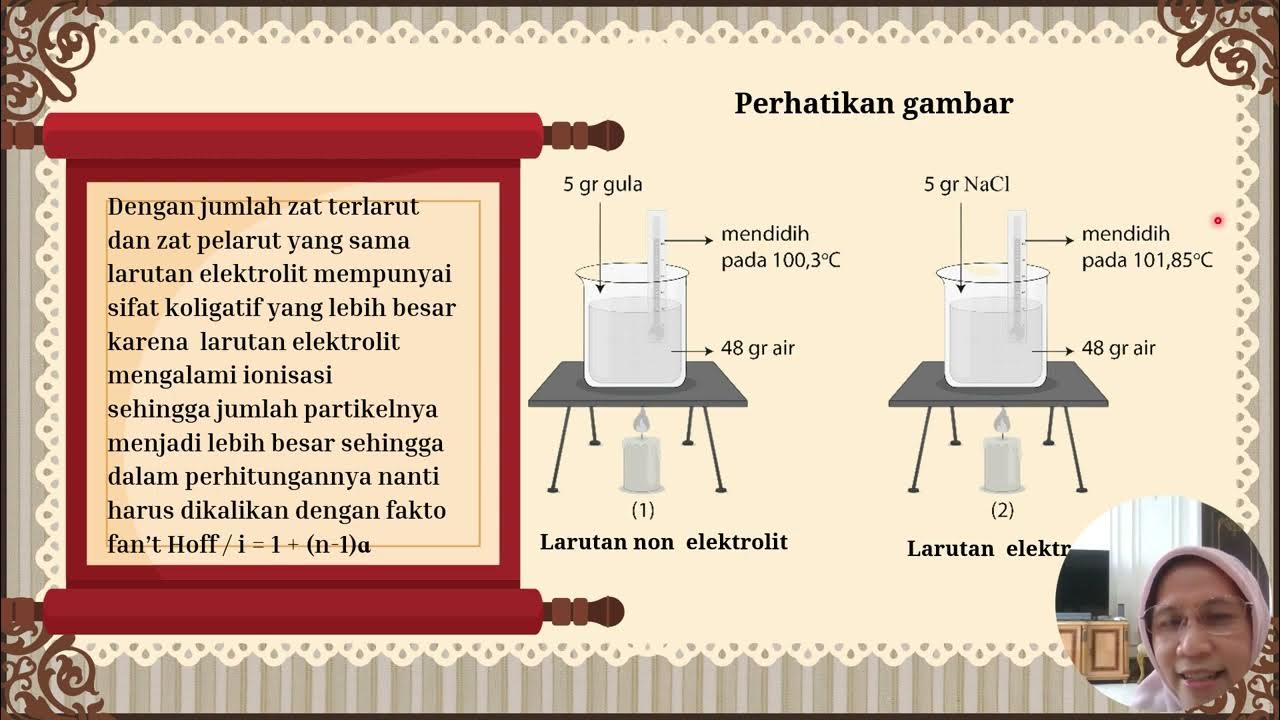
- 😀 The Van't Hoff factor is crucial in calculating the effect of solutes in solutions, especially for electrolytes that dissociate into multiple ions.
- 😀 Strong electrolytes are fully ionized in solution and have a Van't Hoff factor equal to the number of ions they produce.
- 😀 Weak electrolytes only ionize partially, and their Van't Hoff factor depends on the degree of ionization (alpha).
- 😀 Non-electrolytes do not ionize in solution, and their Van't Hoff factor is equal to 1, meaning they do not contribute additional particles to the solution.
Q & A
What are colligative properties of solutions?
-Colligative properties of solutions are properties that depend only on the amount of solute, not on the type of solute or solvent. They are influenced by the number of solute particles present in the solution.
What are the four main colligative properties?
-The four main colligative properties of solutions are: 1) Decrease in saturated vapor pressure, 2) Increase in boiling point, 3) Freezing point depression, and 4) Osmotic pressure.
How does the type of solute affect colligative properties?
-The type of solute does not affect colligative properties. These properties are only influenced by the amount of solute dissolved in the solution, not by the chemical nature of the solute.
What is the Van't Hoff factor, and why is it important?
-The Van't Hoff factor is a quantity used to describe the number of particles into which a substance dissociates in solution. It is important because it helps calculate the effect of solute on colligative properties, particularly for electrolytes and non-electrolytes.
What is the difference between strong and weak electrolytes in terms of colligative properties?
-Strong electrolytes are completely ionized in solution, resulting in a higher Van't Hoff factor. Weak electrolytes are partially ionized, meaning their Van't Hoff factor depends on the degree of ionization (alpha), which is less than 1.
How do non-electrolytes behave in terms of colligative properties?
-Non-electrolytes do not dissociate into ions in solution, so their Van't Hoff factor is 1, meaning the number of dissolved particles equals the number of moles of solute.
What is the difference between molarity and molality?
-Molarity (M) refers to the number of moles of solute per liter of solution, while molality (m) refers to the number of moles of solute per kilogram of solvent. Molality is used when the solution's volume may change with temperature, while molarity is more commonly used in general solutions.
Can you determine the colligative properties of a solution by knowing the type of solute?
-No, the colligative properties of a solution depend only on the amount of solute present, not the type of solute. This means that different solutes with the same quantity can affect properties like boiling point or freezing point in the same way.
What is the significance of the degree of ionization (alpha) for weak electrolytes?
-The degree of ionization (alpha) for weak electrolytes is significant because it affects how much the electrolyte dissociates into ions in solution. A lower alpha means fewer ions, which results in a smaller effect on colligative properties.
What substances are typically considered strong electrolytes?
-Strong electrolytes are substances that fully dissociate into ions in solution. Examples include ionic compounds like NaCl, MgCl2, and KBr, as well as strong acids like HCl and strong bases like NaOH.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)