What is the difference between an Atom, Element, Molecule and Compound?
Summary
TLDRThe video script delves into the fundamental differences between elements, molecules, and compounds, emphasizing the atom as the smallest unit. It explores various types of atomic combinations, such as carbon dioxide and water, and their significance in the universe and life forms. The script also touches on the importance of molecular structures and their roles in the ecosystem, including the formation of elements like iron and gold. The educational content aims to provide insights into the chemical world, encouraging viewers to explore the intricate relationships between atoms and molecules.
Takeaways
- 🔬 Atoms are the smallest unit of matter, making up everything in the universe.
- 🧪 Elements are pure substances made up of only one type of atom, such as hydrogen or oxygen.
- 🔗 Molecules are formed when two or more atoms combine chemically, like H2O (water) or CO2 (carbon dioxide).
- ⚛️ Compounds are substances formed from two or more different elements that are chemically bonded, like NaCl (table salt).
- 🌿 Plants and animals are composed of different elements and compounds, demonstrating the diversity of matter in the universe.
- 🧬 Chemical combinations can be homonuclear (same type of atom) or heteronuclear (different types of atoms).
- 💧 Monoatomic molecules consist of single atoms, like noble gases (Neon, Argon).
- 🧫 Diatomic molecules are composed of two atoms, such as O2 (oxygen) and H2 (hydrogen).
- 🌍 Elements and compounds are fundamental to various natural and synthetic processes, contributing to the diversity of substances.
- 📝 Understanding the basic units of matter, including atoms, elements, molecules, and compounds, is crucial for studying chemistry and the natural world.
Q & A
What is the difference between an atom, a molecule, and a compound?
-An atom is the smallest unit of an element, a molecule is a group of two or more atoms bonded together, and a compound is a substance composed of two or more different types of atoms chemically bonded together.
How are elements different from molecules?
-Elements are pure substances made of only one type of atom, whereas molecules are made up of two or more atoms of the same or different elements bonded together.
What is the chemical formula for water?
-The chemical formula for water is H2O, which indicates that each molecule of water consists of two hydrogen atoms and one oxygen atom.
What is the significance of the term 'elemental' in the context of the script?
-In the script, 'elemental' refers to substances that are composed of a single type of atom, such as elemental hydrogen, oxygen, or carbon.
What is a homoeomolecule and how does it differ from a heteroatom molecule?
-A homoeomolecule is a molecule that consists of atoms of the same element, like O2 (oxygen). A heteroatom molecule contains atoms of different elements, such as H2O (water).
What is the role of carbon, hydrogen, and oxygen in forming organic compounds?
-Carbon, hydrogen, and oxygen are the primary elements in organic compounds. Carbon forms the backbone of most organic molecules, while hydrogen and oxygen are often involved in functional groups that determine the molecule's properties.
How are atoms combined to form molecules?
-Atoms combine to form molecules through chemical bonds, which can be covalent, ionic, or metallic, depending on the types of atoms involved.
What is the significance of the term 'molecular' in the script?
-The term 'molecular' in the script refers to the properties and behaviors of molecules, which are groups of atoms bonded together, and their interactions in various chemical processes.
What is the relationship between atoms and elements?
-Atoms are the basic units of elements. An element is defined by the type of atoms it contains, and all atoms of an element have the same number of protons in their nuclei.
How do different atoms combine to form compounds?
-Different atoms combine to form compounds through chemical bonding, where atoms share, donate, or accept electrons to achieve a stable electron configuration.
What is the importance of understanding atomic combinations in the study of chemistry?
-Understanding atomic combinations is crucial in chemistry as it helps explain the formation of molecules and compounds, their chemical properties, and how they interact with each other.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频

Conceitos básicos [Módulo 01_Aula 04]

Difference between an Atom, a Molecule and a Compound
Elements and atoms | Atoms, compounds, and ions | Chemistry | Khan Academy

Elements vs Compounds (Definitions, Examples, and Practice)

Elements, Atoms, Molecules, Ions, Ionic and Molecular Compounds, Cations vs Anions, Chemistry
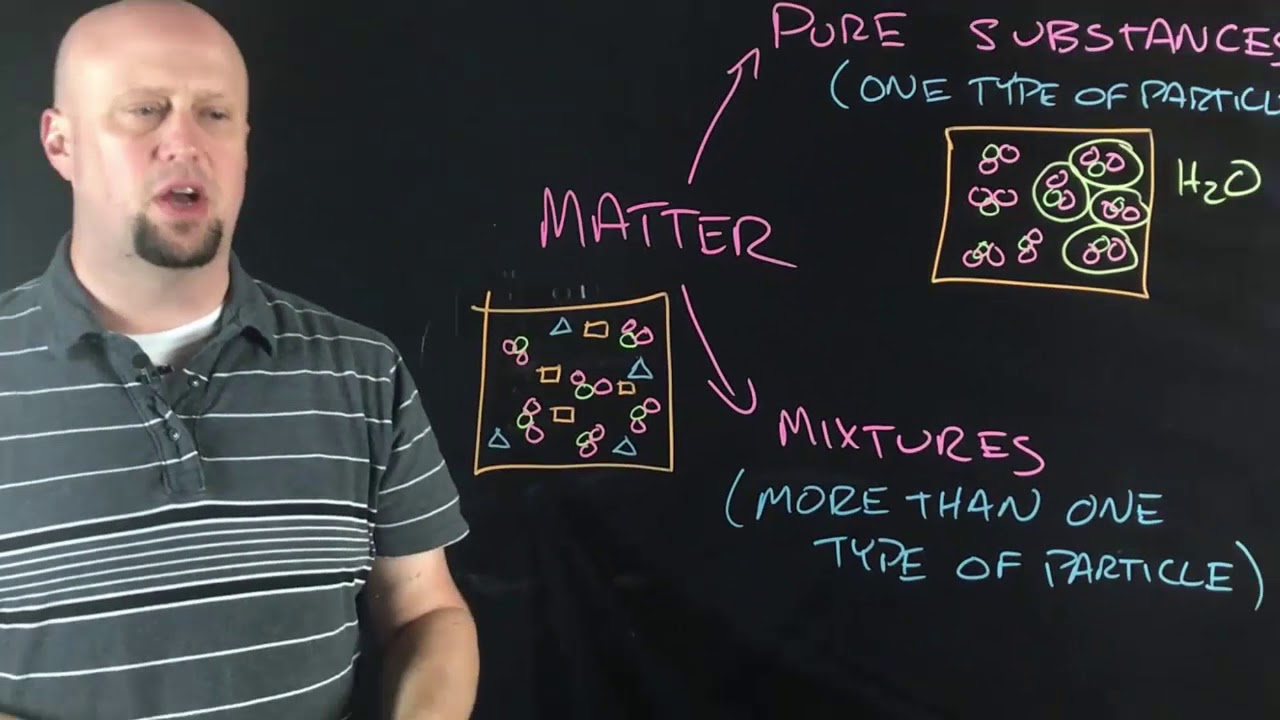
Classification pt 1 Pure Substances
5.0 / 5 (0 votes)
