Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
Summary
TLDRThis video script delves into colligative properties, which depend on solute concentration rather than particle identity. It covers boiling point elevation, freezing point depression, osmotic pressure, and vapor pressure, illustrating how these properties change with solute concentration. The script provides formulas and examples, such as calculating the boiling point of a NaCl solution and the freezing point of an AlCl3 solution, to demonstrate the direct and inverse relationships between solute concentration and these properties, concluding with a problem to determine which solution has the highest boiling point.
Takeaways
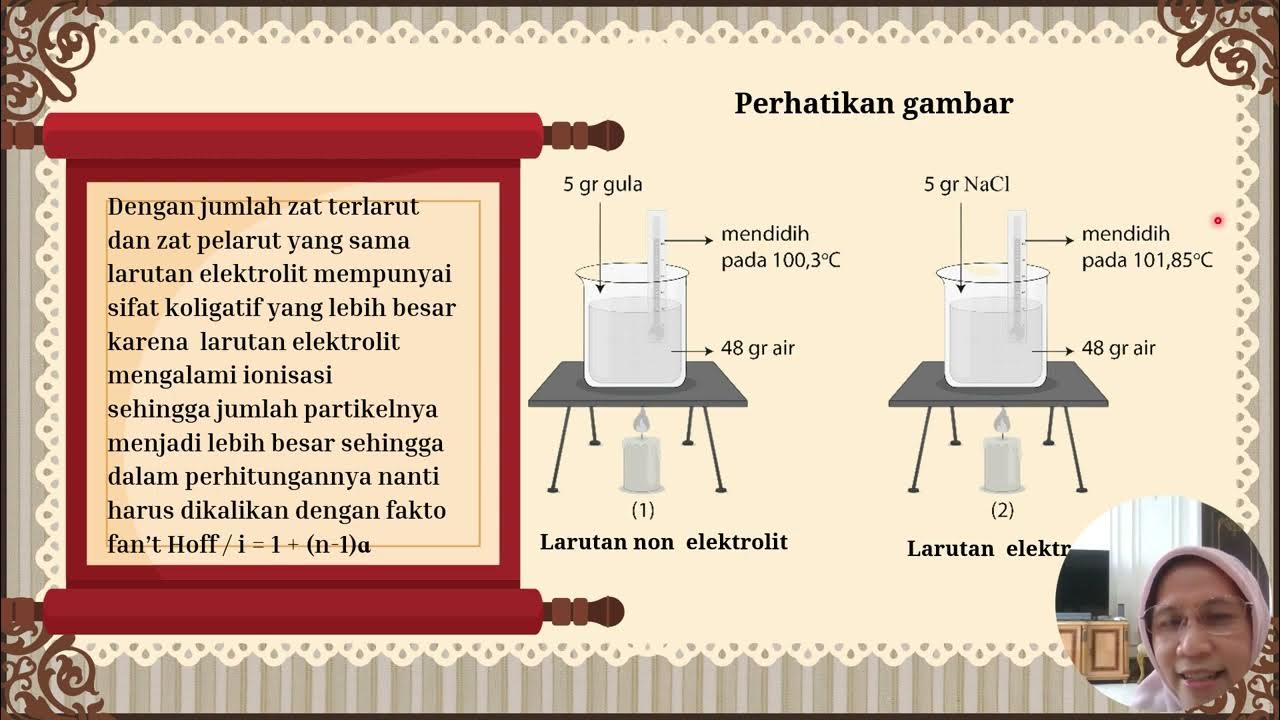
- 🧪 Colligative properties are dependent on the concentration of solute particles in a solution, not the identity of the particles.
- 🔍 The four main colligative properties are boiling point elevation, freezing point depression, osmotic pressure, and vapor pressure.
- 🌡 Boiling point elevation occurs when the addition of solute particles to a solution increases its boiling point.
- ❄️ Freezing point depression happens when the addition of solute lowers the freezing point of the solution.
- 🌪 Osmotic pressure is the pressure that needs to be applied to a solution to prevent the inward flow of solvent through a semipermeable membrane.
- 🌫 Vapor pressure of a solution is inversely related to the concentration of solute particles; as solute concentration increases, vapor pressure decreases.
- 📈 The boiling point elevation and osmotic pressure are directly related to the concentration of solute particles, while freezing point depression and vapor pressure are inversely related.
- 🧊 Adding salt to water, as an example, increases the boiling point of the solution and decreases its freezing point.
- 📚 The formula for calculating the change in boiling point is ΔT = Kb * m * i, where Kb is the boiling point elevation constant, m is molality, and i is the van't Hoff factor.
- ❄️ The formula for calculating the change in freezing point is ΔT = -Kf * m * i, where Kf is the freezing point depression constant, m is molality, and i is the van't Hoff factor.
- 📉 The van't Hoff factor (i) represents the number of ions a solute dissociates into when dissolved in a solution, affecting the magnitude of colligative properties.
- 🛣️ The practical application of freezing point depression is seen in winter when salt is spread on roads to lower the freezing point of water, preventing ice formation.
Q & A
What is a colligative property?
-A colligative property is a property of a solution that depends on the concentration of solute particles in the solution, not on the identity of those particles.
Name the four colligative properties discussed in the script.
-The four colligative properties discussed are boiling point elevation, freezing point depression, osmotic pressure, and vapor pressure.
How does the boiling point of water change when solute particles are added?
-When solute particles are added to water, the boiling point of the solution increases or elevates.
What formula is used to calculate the boiling point of a solution?
-The formula used to calculate the boiling point of a solution is ΔTb = Kb * m * i, where Kb is the boiling point elevation constant, m is the molality, and i is the van't Hoff factor.
What is the van't Hoff factor and why is it important in colligative properties?
-The van't Hoff factor (i) represents the number of ions a solute dissociates into when dissolved in a solution. It is important because it affects the concentration of particles in the solution, influencing colligative properties like boiling point elevation.
How does the freezing point of a solution change with the addition of a solute?
-The freezing point of a solution decreases or is depressed when a solute is added.
What is the relationship between solute concentration and vapor pressure in a solution?
-There is an inverse relationship between solute concentration and vapor pressure. As solute concentration increases, the vapor pressure of the solution decreases.
How is osmotic pressure related to the concentration of solute particles?
-Osmotic pressure has a direct relationship with the concentration of solute particles. As the concentration of solute particles increases, the osmotic pressure also increases.
What is the difference between molality (m) and molarity (M)?
-Molality (m) is the moles of solute per kilogram of solvent, while molarity (M) is the moles of solute per liter of solution.
Why is the freezing point depression property useful in wintertime?
-The freezing point depression property is useful in winter because when salt is added to water, it lowers the freezing point, making it difficult for water to freeze and helping ice to melt, which is why it's used on roads during winter.
How can you calculate the boiling point of a solution with a given solute?
-You can calculate the boiling point of a solution by using the formula for boiling point elevation and knowing the molality of the solution, the boiling point elevation constant (Kb), and the van't Hoff factor.
How can you determine which solution has the highest boiling point among multiple solutions?
-To determine which solution has the highest boiling point, you need to calculate the product of molality (m) and the van't Hoff factor (i) for each solution and compare these values; the solution with the highest mi product will have the highest boiling point.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)