ruangbelajar - Kimia XII SMA - Pendahuluan Sifat Koligatif
Summary
TLDRThis video introduces the concept of colligative properties in solutions, explaining how these properties, including boiling point elevation and freezing point depression, depend solely on the number of solute particles present. The presenter uses relatable examples, such as sugar dissolved in water, to illustrate how solutions become homogeneous mixtures. The video further details mathematical formulas for calculating molarity, molality, and mole fraction, emphasizing the significance of solute concentration in determining a solution's unique characteristics. This engaging overview serves as an essential foundation for understanding the behavior of solutions in chemistry.
Takeaways
- 😀 Solutions consist of a solvent (like water) and a solute (like sugar), forming a homogeneous mixture.
- 📈 Colligative properties are characteristics of solutions that depend on the number of solute particles rather than their identity.
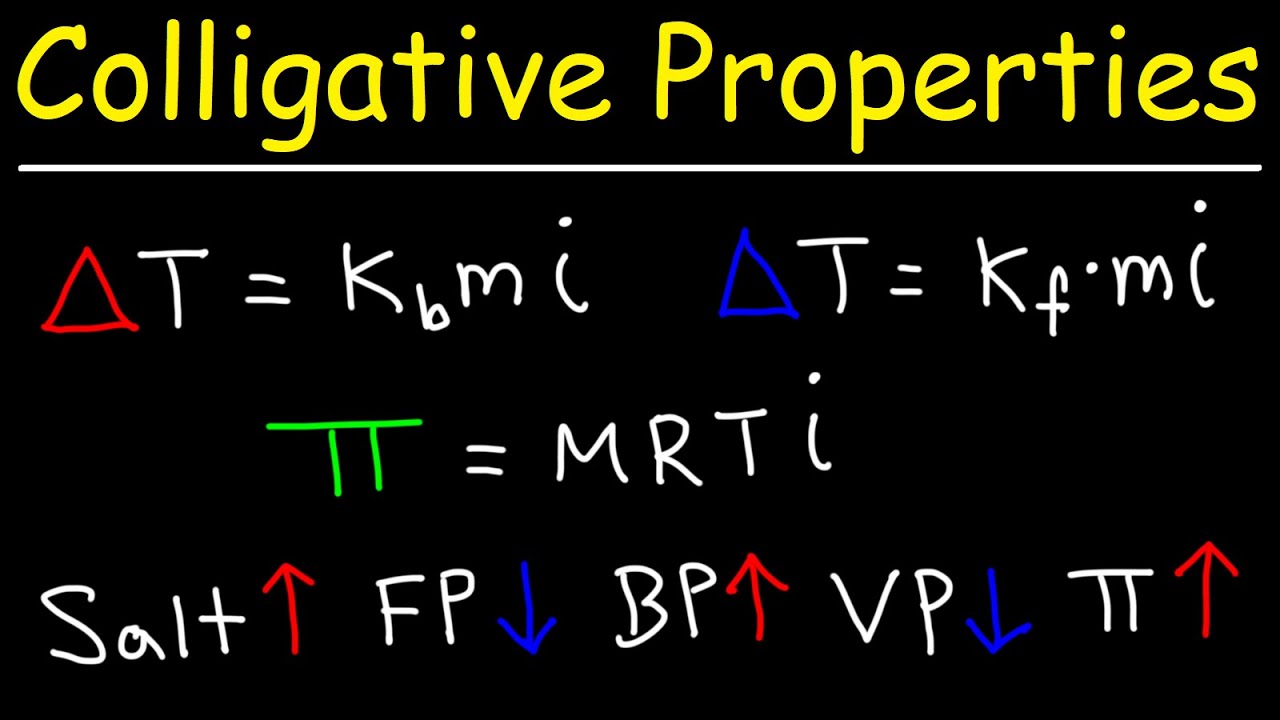
- 🌡️ Boiling Point Elevation (ΔTB) occurs when the boiling point of a solution is higher than that of the pure solvent.
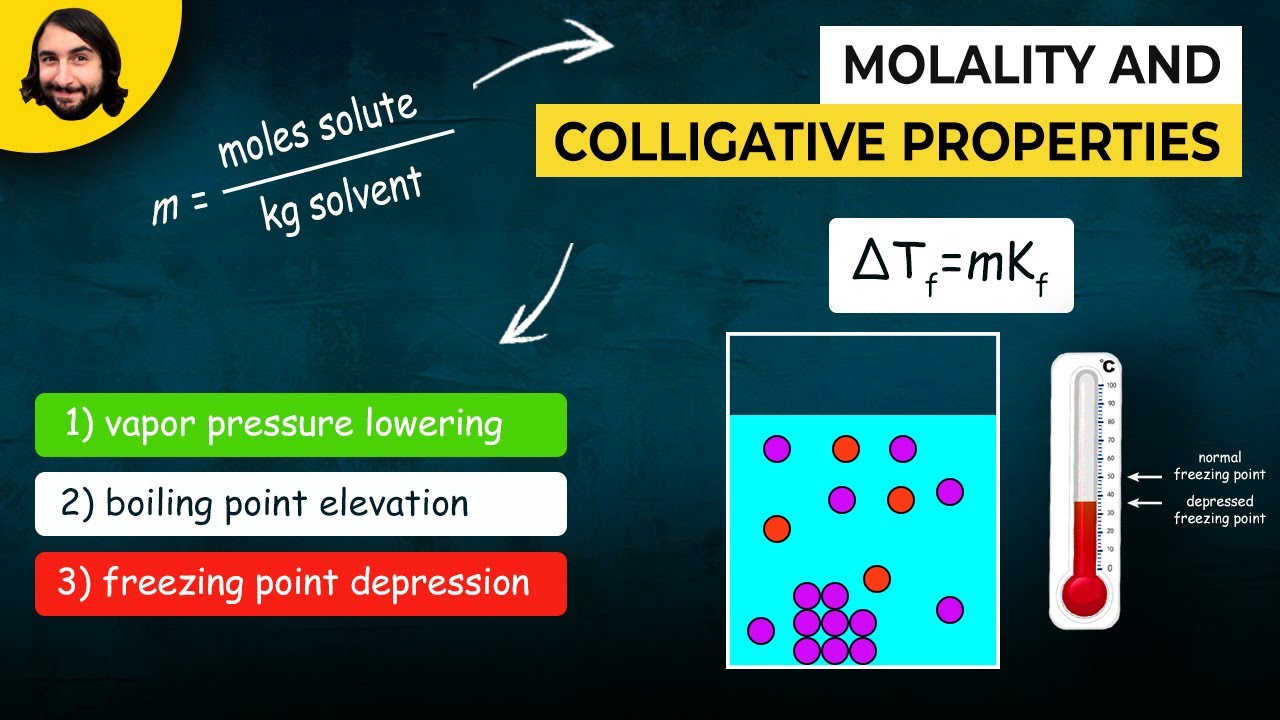
- ❄️ Freezing Point Depression (ΔTF) means the freezing point of a solution is lower than that of the pure solvent.
- 💧 Vapor Pressure Lowering (ΔP) indicates that the presence of a solute reduces the vapor pressure of the solvent.
- 💦 Osmotic Pressure (π) is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane.
- 🔍 The magnitude of colligative properties increases with the number of solute particles present in the solution.
- 📊 Molarity (M), a way to express concentration, is calculated by dividing the mass of solute by the volume of solution in liters.
- ⚖️ Molality (m) measures concentration based on the mass of solvent rather than volume, which is essential for accurate calculations.
- 🔢 Mole Fraction (X) represents the ratio of the number of moles of a component to the total number of moles in the solution.
Q & A
What is a solution, and how is it formed?
-A solution is a homogeneous mixture formed by dissolving a solute in a solvent. For example, when sugar is dissolved in water, the result is a uniform mixture where it's impossible to distinguish between the sugar (solute) and water (solvent).
What are colligative properties?
-Colligative properties are characteristics of solutions that depend on the number of solute particles, not the type of particles. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure.
What is boiling point elevation (ΔT₋B)?
-Boiling point elevation is a colligative property where the boiling point of a solution is higher than that of the pure solvent. This occurs because the presence of solute particles prevents solvent molecules from escaping easily into the gas phase.
How does the number of solute particles affect colligative properties?
-The more solute particles present in a solution, the greater the effect on the colligative properties. For example, adding more solute to a solution will increase its boiling point elevation and decrease its freezing point depression.
What is freezing point depression (ΔT₋F)?
-Freezing point depression is the lowering of the freezing point of a solution compared to the pure solvent. The presence of solute particles disrupts the formation of the solid structure, requiring a lower temperature to freeze the solution.
What is vapor pressure lowering (ΔP)?
-Vapor pressure lowering occurs when the vapor pressure of a solution is lower than that of the pure solvent. This is due to solute particles occupying the surface of the solution, reducing the number of solvent molecules that can evaporate.
What is osmotic pressure (Π)?
-Osmotic pressure is the pressure required to stop osmosis in a solution. It depends on the number of solute particles, and higher solute concentrations result in greater osmotic pressure.
How can you calculate molarity (M) of a solution?
-Molarity (M) is calculated by dividing the number of moles of solute by the volume of solution in liters. The formula is M = (mass of solute / molar mass of solute) × 1000 / volume of solution (in mL).
What is molality (m), and how is it different from molarity?
-Molality (m) measures the number of moles of solute per kilogram of solvent. It differs from molarity because molality uses mass of the solvent, whereas molarity uses the volume of the solution.
How is mole fraction (X) calculated for a solute in a solution?
-Mole fraction (X) is calculated as the ratio of the moles of solute to the total moles of solute and solvent in the solution. For solute, the formula is X = (moles of solute) / (moles of solute + moles of solvent).
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)