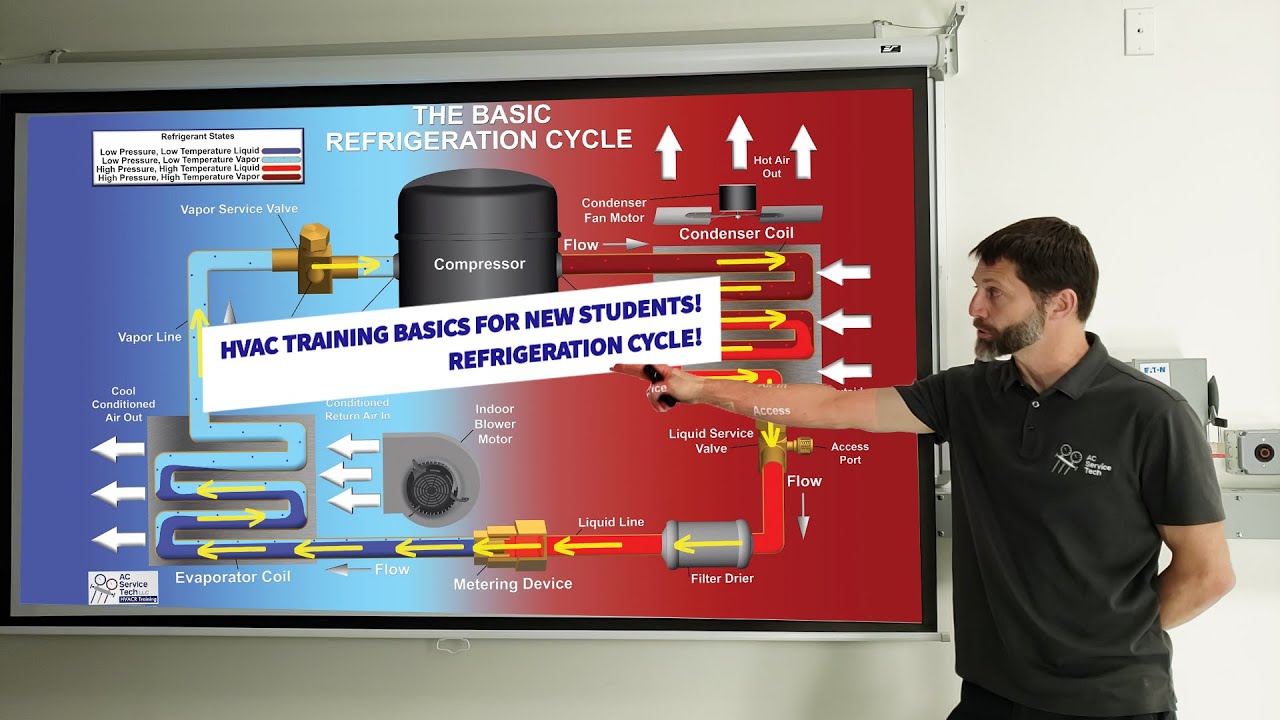
#Refrigerasi- 003Kondensor
Summary
TLDRThis video explains the refrigeration system, focusing on the condenser component. It details the process of refrigerant exiting the compressor with increased temperature and pressure, then entering the condenser where heat is released to the environment. The refrigerant undergoes a phase change from a saturated gas to a liquid, resulting in a decrease in enthalpy. The system is analyzed as an open system, considering the energy entering and exiting through enthalpy and heat transfer. The video concludes with the energy balance equation for the condenser, emphasizing the relationship between mass flow rate, enthalpy, and heat released to the surroundings.
Takeaways
- 😀 The condenser in a refrigeration system is where the refrigerant releases heat to the surrounding environment, transitioning from a superheated gas to a saturated liquid.
- 😀 The refrigerant enters the condenser with high pressure and temperature (T3), and it undergoes a reduction in both temperature and enthalpy during the process.
- 😀 The phase change in the condenser occurs at constant pressure, where the refrigerant condenses from a superheated gas to a saturated liquid.
- 😀 The energy balance in the condenser is analyzed by equating the energy entering the system with the energy leaving it, ensuring no energy is accumulated within the component.
- 😀 The refrigerant's enthalpy decreases from H3 at the inlet to H4 at the outlet of the condenser, reflecting the heat loss during the phase change.
- 😀 The energy entering the condenser is primarily in the form of enthalpy, while the energy leaving includes both the reduced enthalpy and the heat released to the environment.
- 😀 The process involves the release of heat to the surroundings, described as Q_cd, which represents the heat rejected by the refrigerant to the environment.
- 😀 The formula for energy balance in the condenser is given by: mass flow rate * (enthalpy at entry) = mass flow rate * (enthalpy at exit) + heat released to the environment.
- 😀 The pressure at the condenser inlet (P3) remains higher than the pressure at the outlet (P4), but the pressure is constant throughout the condensation process.
- 😀 The refrigerant's mass flow rate, enthalpy, and the heat released to the environment are critical parameters in understanding and analyzing the condenser's performance.
Q & A
What happens to the refrigerant after it exits the compressor?
-After exiting the compressor, the refrigerant experiences a temperature and pressure increase. Its temperature rises from T2 to T3, and its pressure also increases from P2 to P3, which leads to an increase in enthalpy from H2 to H3.
What is the role of the condenser in the refrigeration system?
-The condenser's role is to release the heat carried by the refrigerant to the surrounding environment. As the refrigerant passes through the condenser, its temperature decreases and it undergoes condensation, changing from a saturated gas to a saturated liquid.
Why is the operating temperature of the condenser higher than the surrounding environment?
-The operating temperature of the condenser is higher than the surrounding environment because it needs to transfer heat to the outside. For heat transfer to occur efficiently, the refrigerant inside the condenser must have a higher temperature than the environment.
What happens during the condensation process in the condenser?
-During condensation, the refrigerant releases heat and transitions from a saturated gas to a saturated liquid. This phase change occurs at a constant pressure and results in a reduction in temperature and enthalpy.
What is the relationship between the refrigerant's enthalpy and the condenser's operation?
-The enthalpy of the refrigerant decreases as it undergoes condensation in the condenser. Initially, the refrigerant has a higher enthalpy (H3) as a saturated gas, but after condensation, its enthalpy drops to a lower value (H4) as a saturated liquid.
How is energy analyzed in the condenser?
-Energy in the condenser is analyzed as an open system where the mass flow rate of the refrigerant carries energy in the form of enthalpy. The energy entering the condenser is the enthalpy of the refrigerant at point H3, and the energy leaving is the enthalpy at point H4, along with the heat released to the environment.
What is the equation used to represent the energy balance in the condenser?
-The energy balance in the condenser can be represented by the equation: (mass flow rate * enthalpy at H3) = (mass flow rate * enthalpy at H4) + heat released to the environment (Qcd). This equation shows the relationship between the energy entering and leaving the system.
What is the significance of assuming steady-state conditions in the analysis?
-Assuming steady-state conditions means that there is no accumulation of energy within the system, which simplifies the analysis. It allows the mass flow rate and energy input/output to be considered constant over time, making the energy balance equation applicable.
Why are potential and kinetic energies considered negligible in the condenser analysis?
-Potential and kinetic energies are considered negligible in the condenser analysis because the height difference and the linear velocities of the refrigerant are not significant enough to affect the overall energy balance in this part of the system.
What will be discussed next after the condenser in the refrigeration cycle?
-After the condenser, the next component to be discussed is the expansion valve, which plays a crucial role in regulating the pressure and flow of the refrigerant in the cycle.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)