David Baker (U. Washington / HHMI) Part 1: Introduction to Protein Design
Summary
TLDRIn this lecture, David Baker from the University of Washington discusses the complexities of protein design and structure prediction. He explains the challenges of determining protein structures from amino acid sequences, as well as the inverse problem of designing proteins from scratch. Baker describes how computational methods like Rosetta are used to predict and design proteins with idealized structures. Through practical examples, including the design of globular proteins, repeat proteins, and small disulfide-bonded peptides, he highlights the potential of engineered proteins to solve modern scientific and medical challenges. The lecture emphasizes the powerful capabilities of computational biology and protein design in advancing functional proteins.
Takeaways
- 😀 Protein folding is crucial for biological function, as proteins adopt unique native structures to perform their roles.
- 😀 Each gene in an organism's genome encodes a unique protein, and understanding these proteins' structures and functions is a key research challenge.
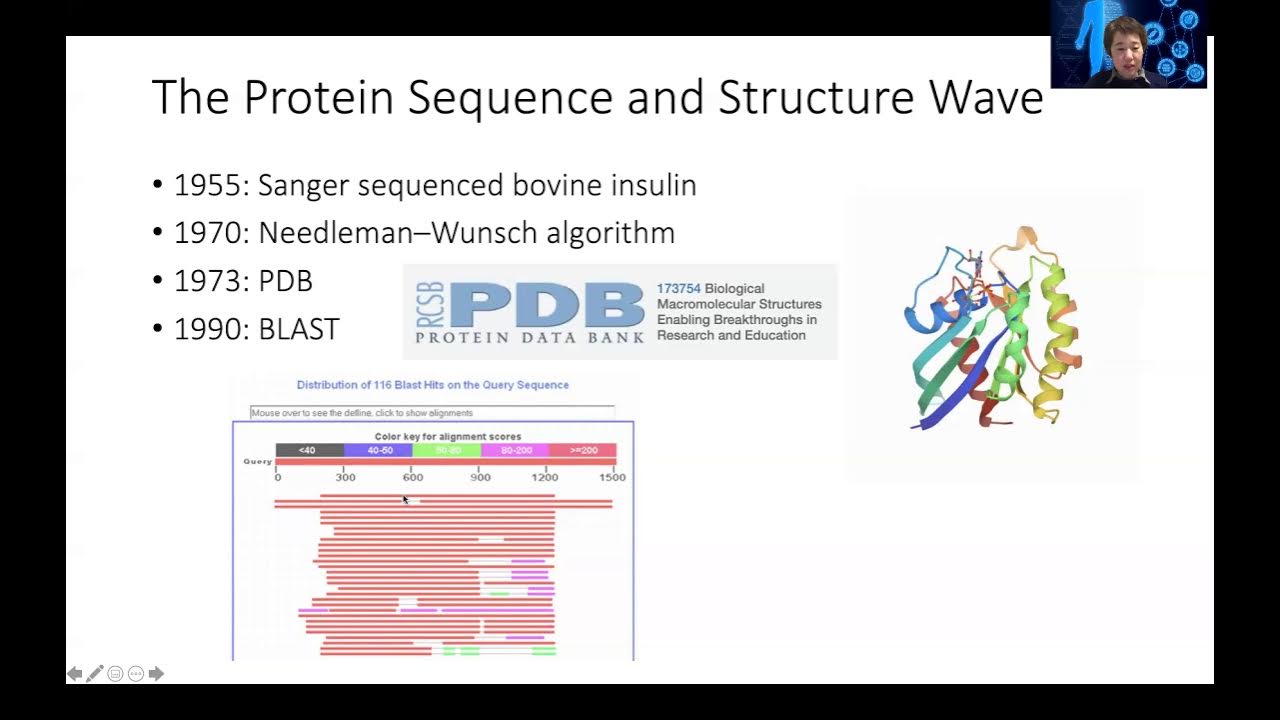
- 😀 The protein structure prediction problem involves predicting the 3D structure of a protein from its amino acid sequence.
- 😀 The protein design problem goes in the opposite direction, where scientists design a sequence of amino acids to form a specific structure.
- 😀 The vast number of possible conformations and sequences makes both the structure prediction and design problems extremely challenging.
- 😀 For a 100-residue protein, there are approximately 3^100 possible conformations, making a brute-force search infeasible.
- 😀 Protein sequence design involves selecting the best amino acid sequence for a given structure by evaluating many possible sequences.
- 😀 Accurate energy calculations are necessary for solving protein design problems, requiring models that consider atomic interactions and environmental factors like water molecules.
- 😀 Rosetta, a computational tool, simulates protein folding and helps identify the lowest-energy structure by sampling possible conformations quickly.
- 😀 Once a designed protein sequence is determined, synthetic DNA is created, and the protein is produced in the lab to test if it folds as intended.
- 😀 Successful protein designs have led to new, idealized proteins, including globular proteins, repeat proteins, and disulfide-bonded proteins, with real-world applications in therapeutics and nanomaterials.
Q & A
What is protein design, and how does it relate to protein structure?
-Protein design is the process of creating new protein structures by determining an amino acid sequence that will fold into a specific desired 3D structure. This contrasts with natural protein folding, where proteins adopt their native structures naturally based on their genetic coding. In protein design, we go backward from a target structure to find the sequence that will fold into it.
Why is predicting protein structure considered a difficult problem?
-Predicting protein structure is difficult because proteins can have an astronomical number of possible conformations due to the flexibility of their amino acid chains. Additionally, the energy landscape of a protein is complex, and calculating the energies accurately is computationally challenging, especially when considering interactions with surrounding water molecules.
How does the Rosetta methodology assist in protein structure prediction?
-The Rosetta methodology helps by simulating the protein folding process on a computer. It quickly samples through possible conformations to identify the lowest energy structure. Rather than evaluating all possible configurations (which is computationally impossible), Rosetta mimics real folding to efficiently find an optimal structure.
What is the role of Rosetta@home in protein design?
-Rosetta@home is a distributed computing project where volunteers' computers run simulations to predict protein structures. These volunteers help identify low-energy structures by running Rosetta calculations. Their work plays a critical role in verifying designed protein sequences and their ability to fold into the target structures.
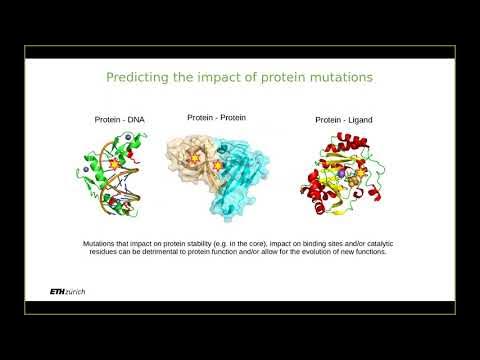
What challenges exist in designing proteins with new, idealized structures?
-Designing proteins with idealized structures presents challenges due to the complexity of finding sequences that will fold perfectly into the desired 3D structures. Additionally, after designing the sequence, it must be synthesized, tested in the lab, and compared to predicted structures, which requires accurate experimental validation.
How do designed proteins differ from naturally occurring proteins?
-Designed proteins are not influenced by evolutionary history or natural selection. They are created as 'ideal' versions, free from the idiosyncrasies and evolutionary adaptations found in naturally occurring proteins. These designed proteins are tailored to meet specific structural and functional goals.
What are some applications of protein design in real-world problems?
-Protein design has various applications, including the creation of novel therapeutics, the development of nanomaterials, and the design of proteins for industrial uses. For instance, small disulfide-bonded proteins may serve as new therapeutics, and repeat proteins could form the basis for nanomaterials like rods and filaments.
What are repeat proteins, and how are they important in protein design?
-Repeat proteins consist of repeating structural modules and can be designed to form large, elongated structures. These proteins are important in protein design because their repetitive nature allows for the creation of large, stable, and predictable structures. They have potential uses in developing new materials and therapeutics.
How are designed proteins manufactured once their sequences are determined?
-Once a protein sequence is designed, it is converted into a DNA sequence that encodes the protein. This DNA is synthesized and introduced into bacteria, which then express the protein. The protein is extracted, purified, and analyzed to verify whether it folds correctly and exhibits the desired properties.
What experimental techniques are used to verify designed protein structures?
-Experimental techniques like nuclear magnetic resonance (NMR) and X-ray crystallography are used to solve and verify the structures of designed proteins. These methods provide detailed atomic-level insights into the protein's 3D structure, allowing comparison with the predicted design.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)