This math trick revolutionized physics
Summary
TLDRThis video delves into Max Planck's pivotal role in shaping modern physics, particularly through his development of quantum mechanics. Faced with the failure of classical physics to explain black body radiation, Planck introduced his energy quantization formula, which maintained the laws of thermodynamics. Initially seen as a mathematical formalism, his work sparked the next leaps in scientific thought, laying the groundwork for the quantum revolution. The script highlights key figures from the era, including Einstein and Madame Curie, and the significance of the 1927 Solvay Conference in advancing quantum theory.
Takeaways
- 😀 Plank’s work on the black body radiation spectrum led to the development of quantum mechanics.
- 😀 Plank was initially hesitant to discard classical physics but was driven to find a solution to the black body radiation problem.
- 😀 In his autobiography, Plank described his approach to solving the problem as an act of desperation due to the fundamental importance of the issue in physics.
- 😀 Plank believed the two laws of thermodynamics must be upheld at all costs, even if it meant sacrificing previous convictions about physical laws.
- 😀 In 1900, Plank introduced his formula for black body radiation, marking the beginning of quantum theory, though he initially viewed his discretization of energy as a mere mathematical trick.
- 😀 Plank did not initially realize the profound implications of his work on the discretization of energy for the future of physics.
- 😀 A key moment in physics history was the 1927 Solvay Conference, which brought together brilliant minds, including Einstein, Bohr, Madame Curie, and others.
- 😀 The first Solvay Conference in 1911 focused on the theory of radiation and quanta, with important figures like Madame Curie, Niels Bohr, and Ernest Rutherford in attendance.
- 😀 The Solvay Conferences played a major role in shaping quantum mechanics, with many notable scientists contributing to its development.
- 😀 The ultimate significance of Plank’s work in 1900 was the creation of the foundation for quantum mechanics, even though it was originally just a mathematical formulation for black body radiation.
Q & A
What was Max Planck's dilemma regarding classical physics?
-Max Planck faced the dilemma of choosing between abandoning classical physics or modifying how entropy was treated. He felt that the problem was of fundamental importance to physics and was determined to find a theoretical interpretation, even if it meant sacrificing his previous convictions about physical laws.
What did Planck think about the two laws of thermodynamics?
-Planck believed that the two laws of thermodynamics should not be altered under any circumstances. Despite the challenges posed by the problem, he was committed to maintaining the integrity of these laws.
What was Planck's approach to solving the problem in 1900?
-Planck's approach involved introducing a mathematical formalism to discretize energy in order to obtain his phenomenological formula for black body radiation. He was unaware of the broader implications of this concept at the time.
What significant event occurred on December 14, 1900?
-On December 14, 1900, Planck presented his final result regarding the black body spectrum at the Berlin Physical Society meeting. This was a pivotal moment in the development of quantum mechanics, even though Planck did not fully realize the consequences of his energy discretization at that point.
How did Planck's work contribute to the development of quantum mechanics?
-Although Planck initially used energy discretization as a mathematical tool, his work laid the groundwork for quantum mechanics. His formula for black body radiation set the stage for other scientists to make the conceptual leap that would lead to the development of quantum theory.
What was the concern of some great physicists regarding Planck's work?
-Some of the greatest physicists of the time were concerned about Planck's introduction of energy discretization, particularly because it challenged the traditional understanding of physics. Despite this, Planck's work ultimately played a foundational role in quantum mechanics.
What is the significance of the famous 1927 Solvay Conference photo?
-The 1927 Solvay Conference photo is iconic because it captured many of the leading scientists of the time, including Albert Einstein, Niels Bohr, Madame Curie, and others. The conference focused on the theory of radiation and quantum mechanics, highlighting the intellectual discussions that shaped modern physics.
Who were some of the notable physicists in the 1927 Solvay Conference photo?
-Notable physicists in the 1927 Solvay Conference photo included Albert Einstein, Niels Bohr, Madame Curie, Werner Heisenberg, and others who contributed significantly to the development of quantum mechanics.
What was the topic of the first Solvay Conference in 1911?
-The first Solvay Conference in 1911, organized by Hendrik Lorentz, focused on the theory of radiation and quanta. It brought together many of the greatest minds of the time to discuss these emerging ideas in physics.
How did Planck's work relate to the ultraviolet catastrophe?
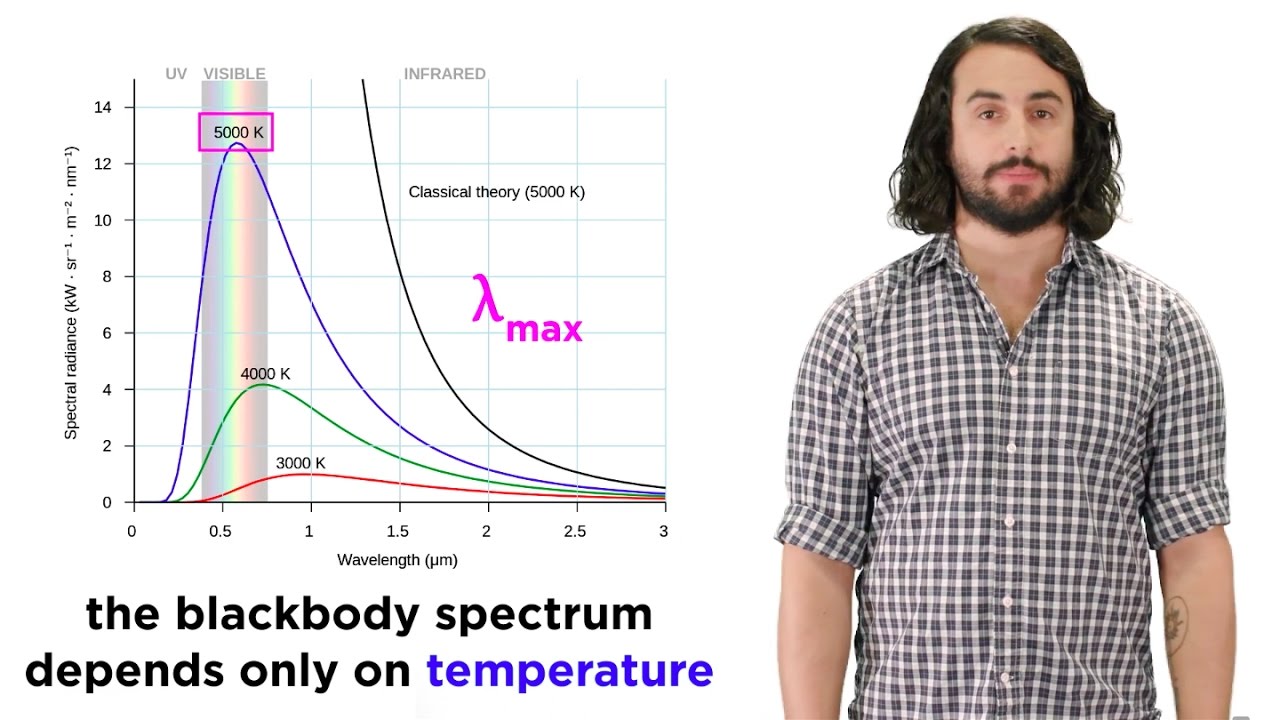
-Planck's work addressed the ultraviolet catastrophe, a problem in classical physics that arose when trying to explain black body radiation. His formula provided a solution by introducing energy quantization, which resolved the issue by preventing infinite radiation at short wavelengths.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

#Fisikapopuler Eps. 1: Sejarah Lahirnya Fisika Kuantum
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe

Quantum Mechanics - Part 1: Crash Course Physics #43

AULA EXTREMAMENTE COMPLETA: Max Planck│ Quantização da Energia │ Modelos Atômicos

Quantization of Energy Part 2: Photons, Electrons, and Wave-Particle Duality

KONSEP DAN FENOMENA KUANTUM - MATERI FISIKA KELAS XII
5.0 / 5 (0 votes)