AULA EXTREMAMENTE COMPLETA: Max Planck│ Quantização da Energia │ Modelos Atômicos
Summary
TLDRThis video lesson delves into Max Planck's pivotal contributions to quantum theory, focusing on energy quantization and his famous equation. The instructor explains how Planck studied the radiation emitted by heated solids, introducing the concept of black-body radiation. Viewers learn how temperature correlates with color and how Planck's formula quantifies energy in discrete multiples. The lesson also highlights the importance of understanding this concept for academic exams and provides exercises for practice. With a mix of theory and practical examples, the video offers a comprehensive, engaging approach to mastering Planck's work and its significance in the evolution of atomic models.
Takeaways
- 😀 Max Planck's work in 1900 revolutionized physics by studying the radiation emitted by heated solids.
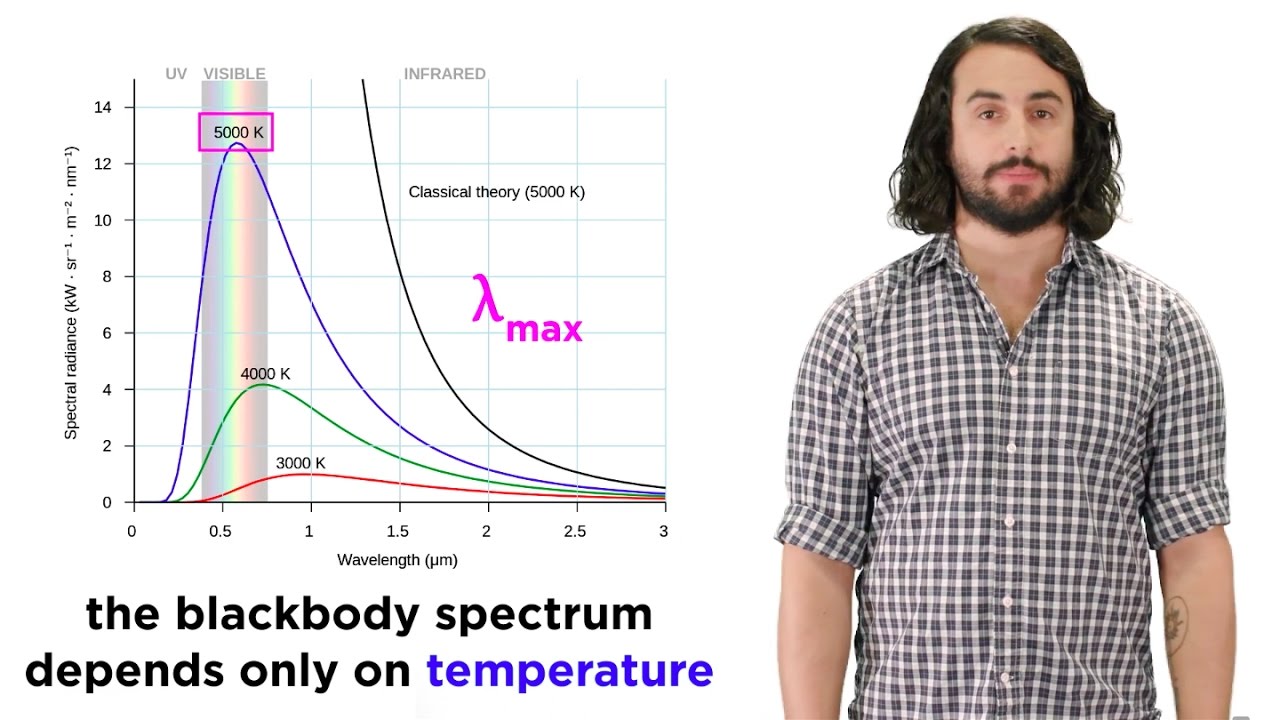
- 😀 Planck discovered that the color and wavelength of the emitted radiation depend on temperature.
- 😀 The concept of 'black body radiation' was key in Planck's research, where a 'black body' absorbs all electromagnetic radiation and emits radiation depending on temperature.
- 😀 In the example of a tungsten filament lamp, the color of the light indicates the temperature, with white being the hottest and red being the coldest.
- 😀 Planck's Quantum Theory introduced the idea of quantized energy levels, where energy is released or absorbed in discrete packets (quanta).
- 😀 The mathematical expression for quantized energy is given by the formula E = h × f, where 'h' is Planck's constant and 'f' is the frequency.
- 😀 Planck's constant (h) is valued at 6.626 × 10^-34 m² kg / s, and it plays a crucial role in determining energy at the quantum level.
- 😀 The frequency (f) of a wave can be calculated using the equation f = v / λ, where v is the wave speed and λ is the wavelength.
- 😀 In quantum mechanics, energy is always released or absorbed in integer multiples of the minimum quantum, denoted by 'hf'.
- 😀 The term 'quantum' refers to the smallest discrete amount of energy, while 'quanta' refers to multiples of these energy units. This concept is central to understanding atomic behavior in quantum theory.
Q & A
What is the main topic discussed in this video?
-The main topic is Max Planck's work on the quantization of energy and the Planck equation, which helped in understanding the energy emission of heated solids and the concept of black body radiation.
Why does the lecturer mention the importance of understanding the Planck equation for future atomic models?
-The lecturer emphasizes that Planck's contributions were crucial in the development of quantum mechanics, which later influenced the creation of atomic models by explaining how energy is emitted or absorbed in discrete quantities.
What is the concept of a black body in relation to Planck's studies?
-A black body is an idealized object that absorbs all electromagnetic radiation that strikes it, and Planck studied the radiation emitted by such bodies, which helped him develop his equation for energy quantization.
What does the color of an object indicate about its temperature in Planck's studies?
-The color of an object reveals its temperature, with white being the hottest, followed by yellow, red, and other colors indicating lower temperatures. This concept is used to understand the radiation emitted by heated solids.
What is the significance of the experiment involving a tungsten filament lamp?
-The lamp serves as a practical example of black body radiation, demonstrating how a tungsten filament emits different colors based on temperature. This visually illustrates Planck's concepts of radiation and energy quantization.
How does the video explain the relationship between electric current and heat in a tungsten filament lamp?
-The video describes how electric current passing through the tungsten filament generates heat, a phenomenon known as Joule's effect, where kinetic energy of electrons is converted into thermal energy, causing the filament to glow.
What is the mathematical expression for Planck's equation?
-The Planck equation is expressed as E = h * f, where E is the energy, h is Planck's constant, and f is the frequency of the radiation. This equation quantizes energy in discrete units.
What is the meaning of 'quantization of energy' in the context of Planck's work?
-Quantization of energy means that energy is not continuous but rather exists in discrete packets, or quanta, where each quantum is a multiple of a fundamental unit, HF, where H is Planck's constant and F is the frequency.
What does the term 'quanta' refer to in the context of energy?
-'Quanta' refers to the discrete packets of energy emitted or absorbed by atoms, which can only take on specific integer multiples of a minimum energy amount, denoted as HF.
What is the role of Planck's constant (h) in his equation?
-Planck's constant (h) is a fundamental physical constant that relates the energy of a photon to its frequency. Its value is approximately 6.626 x 10^-34 J·s, and it is essential for the quantization of energy in Planck's equation.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

This math trick revolutionized physics

Quantum Mechanics: Three Foundational Papers – Planck (1900), Einstein (1905), Bohr (1913)
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe

Struktur Elektron Atom

GATE XL | Chemistry | Lecture 1 | Gurmantra Academy

#Fisikapopuler Eps. 1: Sejarah Lahirnya Fisika Kuantum
5.0 / 5 (0 votes)