Understanding Conduction and the Heat Equation
Summary
TLDRThis video explores the science of thermal conduction, explaining how heat transfers through materials from hot to cold regions via molecular vibrations and free electrons. It covers the fundamental concepts of thermal conductivity, heat transfer rates, and Fourier's law. The video also dives into the heat equation, showing how heat spreads through materials over time and space. Examples include heat transfer through a wall, as well as material properties like thermal diffusivity. The video wraps up with a discussion on using numerical methods and software for solving complex heat transfer problems.
Takeaways
- 😀 Thermal conduction occurs when heat flows from hotter to colder areas within a material due to the random motion of atoms and molecules.
- 😀 The atomic lattice structure and the stiffness of atomic bonds determine how easily heat is transferred through a material.
- 😀 Metals conduct heat well because of free electrons that move through the lattice, in addition to atomic vibrations.
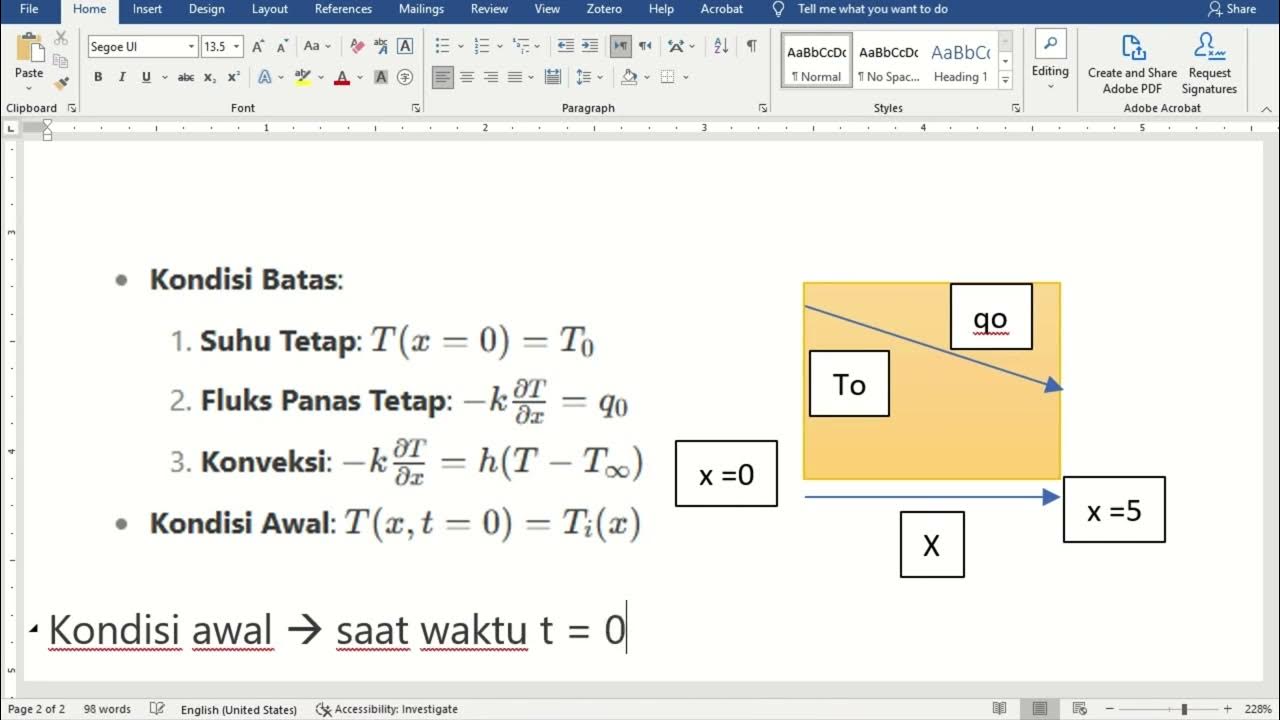
- 😀 The rate of heat transfer through a material can be calculated using Fourier's Law, which depends on temperature gradient, material area, and thermal conductivity.
- 😀 Thermal conductivity (k) is a material property that determines how well heat is conducted and is measured in Watts per meter-Kelvin.
- 😀 Gases and non-metallic liquids have low thermal conductivities due to the large spacing between molecules, while metals are excellent conductors.
- 😀 Diamond, despite not being metallic, has high thermal conductivity due to its regular crystalline structure and strong atomic bonds.
- 😀 Fourier’s Law can also be applied to two- and three-dimensional heat transfer scenarios, where heat flows perpendicular to isotherms (lines or surfaces of constant temperature).
- 😀 The Heat Equation helps engineers determine the temperature distribution across a material over time and space by balancing thermal energy flow and storage.
- 😀 Thermal diffusivity (α) describes how quickly heat spreads through a material and is determined by the ratio of thermal conductivity to volumetric heat capacity.
- 😀 In complex scenarios, numerical methods and software are used to solve the Heat Equation, while thermal resistance can simplify analyses, especially in multi-layer systems.
Q & A
What is thermal conduction and why is it important in engineering?
-Thermal conduction is the process of heat transfer through a material, where thermal energy moves from areas of higher temperature to lower temperature. It is crucial in engineering for designing systems that manage heat, such as in car brakes, building insulation, and electronics.
How does temperature affect the motion of atoms and molecules in a material?
-As temperature increases, the motion of atoms and molecules becomes more significant, with their random motion increasing. At absolute zero, atomic motion is minimal, and the material is in a state of minimal thermal energy.
What role do atomic bonds and lattice structures play in heat conduction?
-Atomic bonds and lattice structures facilitate the transfer of thermal energy through vibrations of atoms. The stiffer and more regular the bonds and structure, the more efficiently heat is conducted.
Why are metals generally better conductors of heat compared to other materials?
-Metals are excellent conductors due to the presence of free electrons that can travel through the material, colliding with other particles and transferring energy. This, along with the vibrations of the atomic lattice, enhances heat conduction.
What is Fourier's Law and how is it used to calculate heat transfer?
-Fourier's Law describes how heat transfers through a material, stating that the heat transfer rate (q) is proportional to the temperature gradient, the material's thermal conductivity, and the surface area. It helps engineers calculate how much energy is lost or gained through walls or other surfaces.
How does thermal conductivity vary across different materials?
-Thermal conductivity varies based on the material's structure. Gases and non-metallic liquids have low thermal conductivity due to the large spacing between molecules, while metals and alloys conduct heat more efficiently. Materials like diamond have high conductivity due to their regular lattice and strong atomic bonds.
What is the difference between heat transfer in one, two, and three dimensions?
-In one-dimensional heat transfer, heat flows in a single direction. In two and three dimensions, heat flow becomes more complex, where the temperature field depends on multiple coordinates (x, y, z), and heat must always flow perpendicular to isotherms (lines or surfaces of constant temperature).
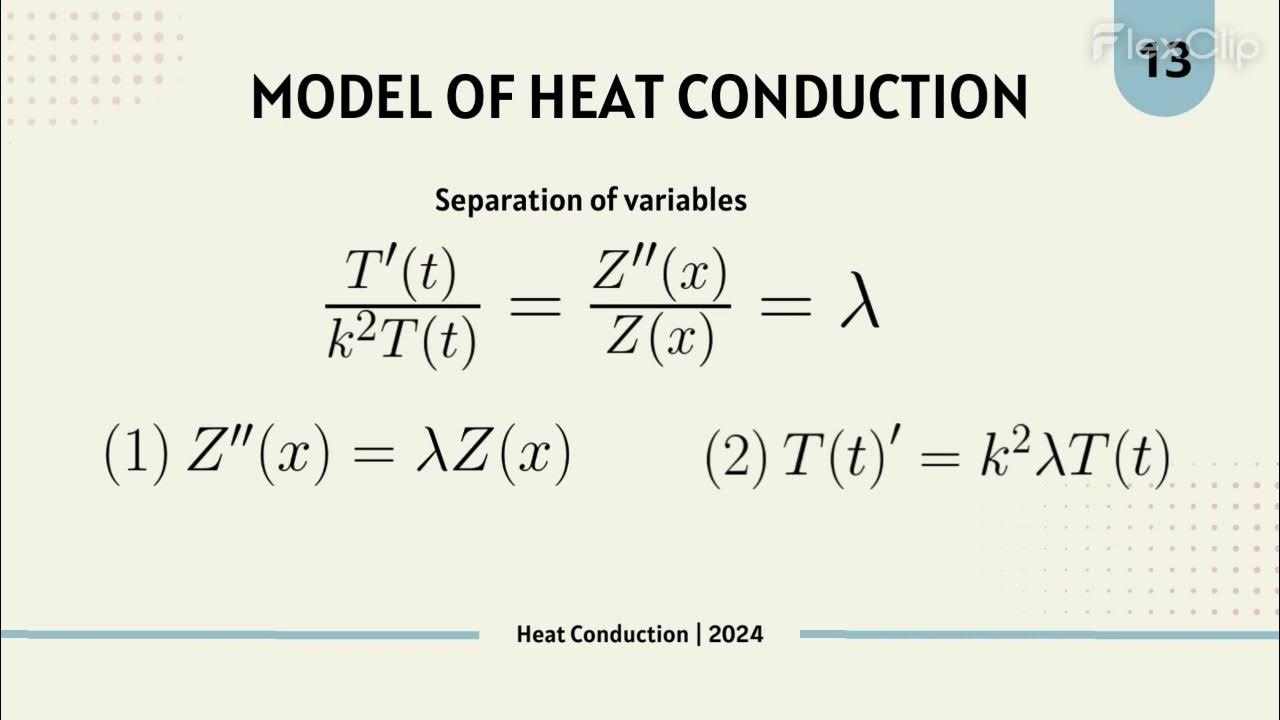
What is the Heat Equation and why is it important in conduction analysis?
-The Heat Equation is a partial differential equation that describes how heat flows throughout an object. It is essential for determining the temperature distribution in more complex systems where heat conduction cannot be directly calculated using Fourier's Law.
How do material properties like density and specific heat capacity influence heat conduction?
-Material properties like density and specific heat capacity affect how heat is stored and transferred. A material’s density and specific heat capacity determine its ability to absorb and store thermal energy, influencing how quickly it responds to temperature changes.
What is thermal diffusivity and how does it relate to heat conduction?
-Thermal diffusivity is a material property that combines thermal conductivity and heat capacity. It represents how quickly heat spreads through a material, with high thermal diffusivity indicating faster heat transfer and lower temperature change per unit of heat.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
5.0 / 5 (0 votes)