Percobaan Elektrolisis KI | Lab Kimia | UPT Laboratorium UHO
Summary
TLDRIn this educational video on electrolysis, viewers are guided through a chemistry experiment where students learn about chemical reactions facilitated by electric currents. The experiment involves using a 0.25 M KCl solution in a transparent tube, connected to a power supply of 6 volts. Participants observe oxidation at the anode and reduction at the cathode, recording changes during the process. The addition of phenolphthalein and FeCl3 further illustrates chemical reactions in the setup. The video concludes with a call for understanding and engagement in future experiments, making complex concepts accessible and intriguing for learners.
Takeaways
- 🔬 The experiment focuses on the process of electrolysis and its chemical reactions driven by electric current.
- ⚗️ Essential equipment includes carbon electrodes, a power supply, and a reaction tube.
- 💧 The solutions used are 0.25 molar KCl and FeCl3, along with phenolphthalein as an indicator.
- ⚡ Electrolysis involves oxidation reactions at the anode and reduction reactions at the cathode.
- 📏 The procedure begins with filling the reaction tube with KCl solution up to 2 cm from the top.
- 🔌 Electrodes are inserted into the tube and connected to a 6-volt DC power supply for 5 minutes.
- 📝 Observations are recorded for changes occurring at the anode and cathode during the experiment.
- 💡 After the initial setup, a 2 ml sample from the cathode is taken and tested with phenolphthalein and FeCl3.
- 🌈 The experiment also involves shaking 1 ml of CHCl3 with the solution from the anode to observe color changes.
- 📊 Participants are encouraged to document all results and write the relevant chemical reaction equations.
Q & A
What is the main objective of the electrolysis experiment?
-The main objective is to study chemical reactions caused by electric current through electrolysis.
What materials are used in the experiment?
-The materials include carbon electrode tubes, a power supply, reaction tubes, a 0.25 M KCl solution, a 0.1 M FeCl3 solution, phenolphthalein indicator, and CHCl3.
What is the definition of electrolysis?
-Electrolysis is a chemical reaction that occurs when an electric current passes through an electrolyte.
What are the roles of the anode and cathode in the electrolysis process?
-The anode is where oxidation occurs, and the cathode is where reduction takes place during electrolysis.
What steps are involved in the experimental procedure?
-The procedure includes filling the reaction tube with KCl solution, connecting the electrodes to a power supply, observing changes, and performing specific reactions with phenolphthalein and FeCl3.
How long should the power supply be connected during the experiment?
-The power supply should be connected for 5 minutes.
What should be observed and recorded during the experiment?
-Changes occurring in the anode and cathode chambers should be observed and documented.
What color change is expected when adding phenolphthalein to the cathode solution?
-Phenolphthalein changes color in response to pH levels; it typically turns pink in a basic solution.
What is the significance of the CHCl3 layer in the experiment?
-The CHCl3 layer is used to observe the color change resulting from the reaction with the solution taken from the anode.
What is the overall conclusion of the electrolysis experiment?
-The experiment illustrates the principles of electrolysis and its practical applications in chemical reactions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

PRAKTIKUM ELEKTROLISIS

Praktikum Sel Elektrolisis Sederhana

Praktikum Konsep Mol (Hukum Kekekalan Massa)

The Whole of AQA - CHEMICAL CHANGES. GCSE 9-1 Chemistry or Combined Science Revision Topic 4 for C1
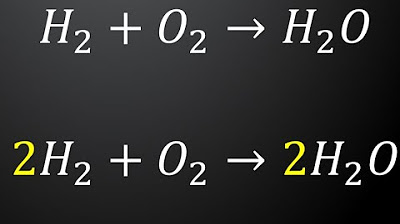
Chemistry: Balancing Chemical Equations (Tagalog Explained)

Video Pembelajaran Model Problem Based Learning Materi Persamaan Reaksi Kimia
5.0 / 5 (0 votes)