Praktikum Konsep Mol (Hukum Kekekalan Massa)
Summary
TLDRIn this educational video, Setyaningrum, a chemistry student, invites 10th-grade high school students to perform a simple chemistry experiment that demonstrates the law of conservation of mass, as proposed by Antoine Lavoisier. The experiment involves mixing water, Betadine, and Vitamin C in a sealed bottle while measuring mass before and after the chemical reaction. The results show that the mass remains constant, proving Lavoisier’s theory. This hands-on experiment emphasizes the importance of scientific laws and offers an accessible way for students to learn about chemistry in everyday settings.
Takeaways
- 😀 Introduction to the experiment: The experiment is aimed at high school students (grade 10) to demonstrate a simple chemistry reaction.
- 😀 Objective: The goal is to demonstrate and validate the Law of Conservation of Mass through a simple chemical reaction.
- 😀 Materials required: Digital scale, sealed bottle, water, Betadine, and vitamin C.
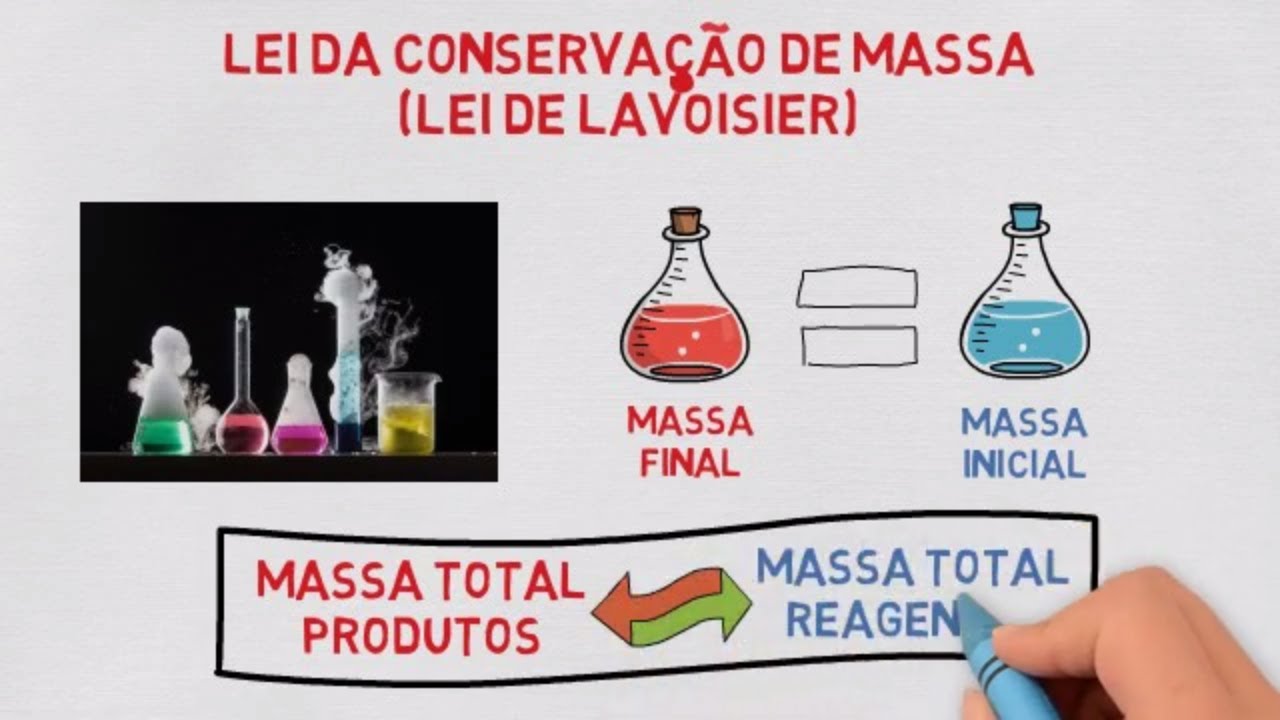
- 😀 Brief explanation of Lavoisier’s Law: The Law of Conservation of Mass states that the mass of substances remains constant before and after a chemical reaction in a closed system.
- 😀 Preparation: Start by preparing all the necessary tools and materials for the experiment.
- 😀 Calibration: Set the digital scale to zero before starting the weighing process to ensure accurate readings.
- 😀 Weighing vitamin C: Measure and record the weight of the vitamin C that will be used in the experiment.
- 😀 Sealed bottle on scale: Place a sealed bottle on the digital scale and set it to zero to account for the weight of the bottle.
- 😀 Adding substances: Add water to the bottle, then Betadine, causing a visible color change, and record the weight.
- 😀 Chemical reaction: Add the vitamin C to the bottle, which turns the solution clear, indicating a chemical reaction (redox reaction).
- 😀 Result: The weight before and after the reaction remained consistent, supporting the Law of Conservation of Mass.
- 😀 Conclusion: The experiment successfully demonstrated that the mass of substances does not change during a chemical reaction when conducted in a closed system.
Q & A
What is the main objective of the chemical experiment presented in the video?
-The main objective of the chemical experiment is to demonstrate the law of conservation of mass, as proposed by Antoine Lavoisier, through a simple chemical reaction.
Who is Antoine Lavoisier and why is he significant in chemistry?
-Antoine Lavoisier was a French chemist in the 18th century, known for discovering the law of conservation of mass, which states that mass is conserved in a chemical reaction within a closed system.
What materials are needed for the chemical experiment?
-The materials required for the experiment include a digital scale, a closed bottle, water, betadine, and vitamin C.
What is the first step in conducting the experiment?
-The first step is to prepare the necessary materials and ensure the digital scale is properly set to zero.
Why is it important to reset the digital scale to zero?
-Resetting the scale to zero is necessary to ensure accurate measurements by accounting for the weight of the bottle before adding any substances.
What happens when vitamin C is added to the betadine solution?
-When vitamin C is added to the betadine solution, the brown color of the betadine disappears, indicating that a chemical reaction (a redox reaction) has occurred.
What is the purpose of weighing the bottle before and after adding ingredients?
-Weighing the bottle before and after adding ingredients helps demonstrate the principle of mass conservation by showing that the mass remains constant before and after the chemical reaction.
What observation indicates that a chemical reaction has occurred in the experiment?
-The observation that the color of the betadine changes from brown to clear indicates that a chemical reaction has occurred, confirming the interaction between vitamin C and betadine.
What was the mass of the substances before and after the reaction?
-Before the reaction, the mass of the water mixed with betadine was 297 grams, and the mass of the vitamin C was 1 gram. After the reaction, the total mass became 298 grams, demonstrating the conservation of mass.
How does this experiment validate the law of conservation of mass?
-The experiment shows that the total mass of the system remained the same before and after the reaction, validating the law of conservation of mass as stated by Lavoisier.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Hukum Dasar Kimia (Hukum Kekekalan Massa/Hukum Lavoisier) – [Kimia X Semester 2]

Hukum Kekekalan Massa - Hukum Dasar Kimia
Lei de Lavoisier: Lei de Conservação das Massas!

Hukum Lavoisier (Hukum Kekekalan Massa) | Kimia SMA | Tetty Afianti

Esperimento legge di Lavoisier

Hukum hukum Dasar Kimia bag 1, XMIPA
5.0 / 5 (0 votes)