Sifat Periodik Unsur | KIMIA SMA | Herlina
Summary
TLDRIn this engaging chemistry lesson, the teacher introduces periodic properties of elements, emphasizing the relationships between atomic number and characteristics like atomic radius, ionization energy, electron affinity, and electronegativity. The lesson explores how these properties change across periods and groups in the periodic table, highlighting the impact of atomic structure on reactivity and properties of metals and nonmetals. The teacher effectively illustrates these concepts with examples, making complex topics accessible and relatable to students, fostering a deeper understanding of elemental behavior in chemistry.
Takeaways
- 😀 Periodic properties of elements change systematically with increasing atomic number.
- 😀 Atomic radius increases down a group due to additional electron shells, while it decreases across a period as effective nuclear charge increases.
- 😀 Ionization energy is the energy required to remove an electron from an atom, which decreases down a group and increases across a period.
- 😀 Electron affinity refers to the energy change when an electron is added to a neutral atom, generally decreasing down a group and increasing across a period.
- 😀 Electronegativity is the tendency of an atom to attract electrons in a bond, decreasing down a group and increasing across a period.
- 😀 Metals, found on the left side of the periodic table, tend to lose electrons and form positive ions, while nonmetals on the right tend to gain electrons and form negative ions.
- 😀 The reactivity of elements varies, with alkali metals being highly reactive and noble gases being mostly inert due to their stable electron configurations.
- 😀 The properties of elements are influenced by their position in the periodic table, with trends observable in atomic size, ionization energy, and electronegativity.
- 😀 Understanding periodic trends is essential for predicting the behavior and reactivity of different elements.
- 😀 The lesson underscores the importance of systematic study in chemistry, linking theoretical concepts with real-world applications.
Q & A
What is the primary focus of the chemistry lesson?
-The primary focus of the chemistry lesson is to analyze the periodic properties of elements, including atomic radius, ionization energy, electron affinity, and electronegativity.
How is atomic radius defined and what factors influence it?
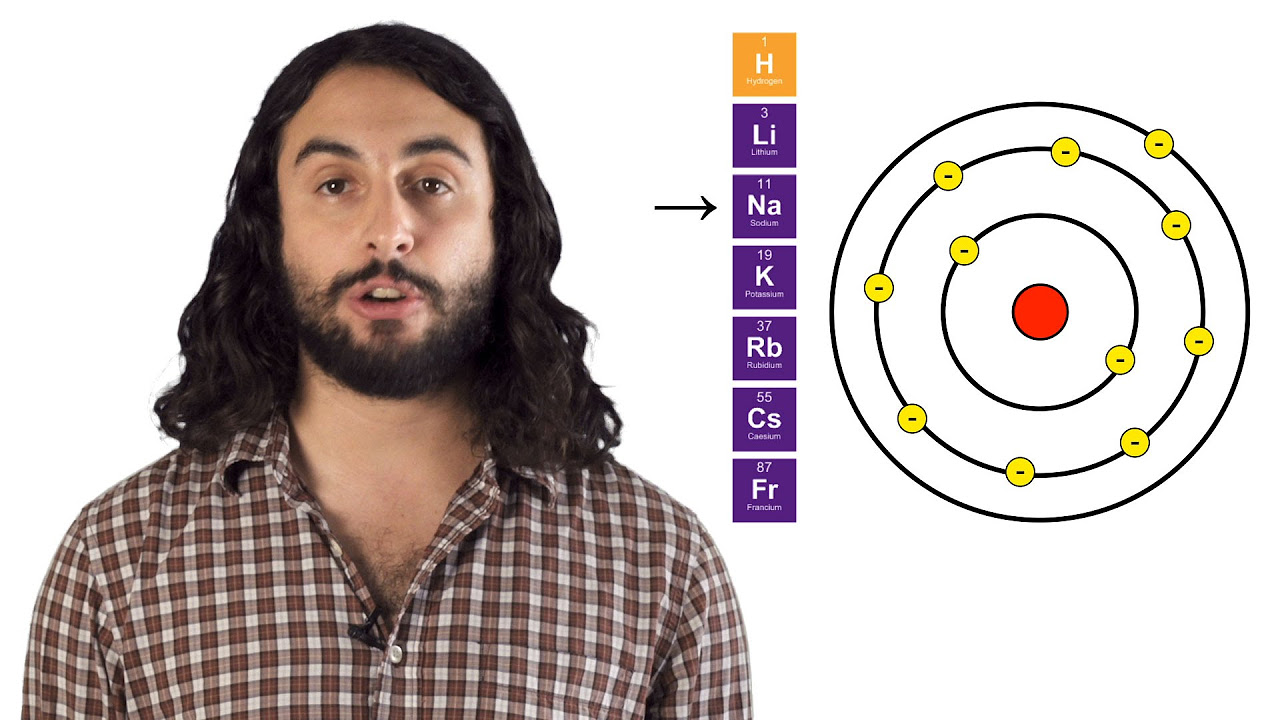
-Atomic radius is defined as the distance from the nucleus to the outermost electron shell. It is influenced by the number of electron shells and the nuclear charge, with larger atomic radii as the number of shells increases and smaller radii across a period due to increased nuclear attraction.
What trend does ionization energy follow across a period and down a group?
-Ionization energy generally increases across a period due to stronger nuclear attraction and decreases down a group because the outermost electrons are further from the nucleus, making them easier to remove.
What is electron affinity and how does it vary in the periodic table?
-Electron affinity is the energy change when an electron is added to a neutral atom. It typically decreases down a group and increases across a period, except for alkaline earth metals and noble gases, which have positive affinities.
Explain electronegativity and its periodic trends.
-Electronegativity measures an atom's ability to attract electrons in a chemical bond. It decreases down a group and increases across a period, indicating that atoms with higher electronegativity are more effective at attracting electrons.
What defines the metallic and nonmetallic properties of elements?
-Metallic properties are characterized by electropositivity, which increases down a group and decreases across a period. Nonmetallic properties, on the other hand, display the opposite trend.
How does reactivity change among different groups of elements?
-Reactivity varies among different groups; for example, alkali metals are highly reactive, while noble gases are non-reactive due to their stable electron configurations.
What is the relationship between atomic radius and the number of electron shells?
-The atomic radius increases with the number of electron shells. As more shells are added, the distance from the nucleus to the outermost electrons becomes greater, resulting in a larger atomic radius.
Why do halogens have a high electron affinity?
-Halogens have a high electron affinity because they are one electron short of having a full outer shell, making them very eager to gain an electron and form negatively charged ions.
What factors determine the strength of attraction between the nucleus and outer electrons?
-The strength of attraction between the nucleus and outer electrons is determined by the nuclear charge (number of protons in the nucleus) and the distance of the outer electrons from the nucleus. A greater nuclear charge and shorter distance lead to a stronger attraction.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Entenda a TABELA PERIÓDICA em 10 minutos - Toda Matéria

SISTEM PERIODIK UNSUR [Sifat Sifat Periodik Unsur]

Sistem Periodik Unsur • Part 6: Sifat Keperiodikan Unsur
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity

Sifat Periodik Unsur | Jari jari Atom | Energi Ionisasi | Afinitas Elektron | Elektronegativitas

Sifat Keperiodikan Unsur | Kimia SMA | Tetty Afianti
5.0 / 5 (0 votes)