Sifat Keperiodikan Unsur | Kimia SMA | Tetty Afianti
Summary
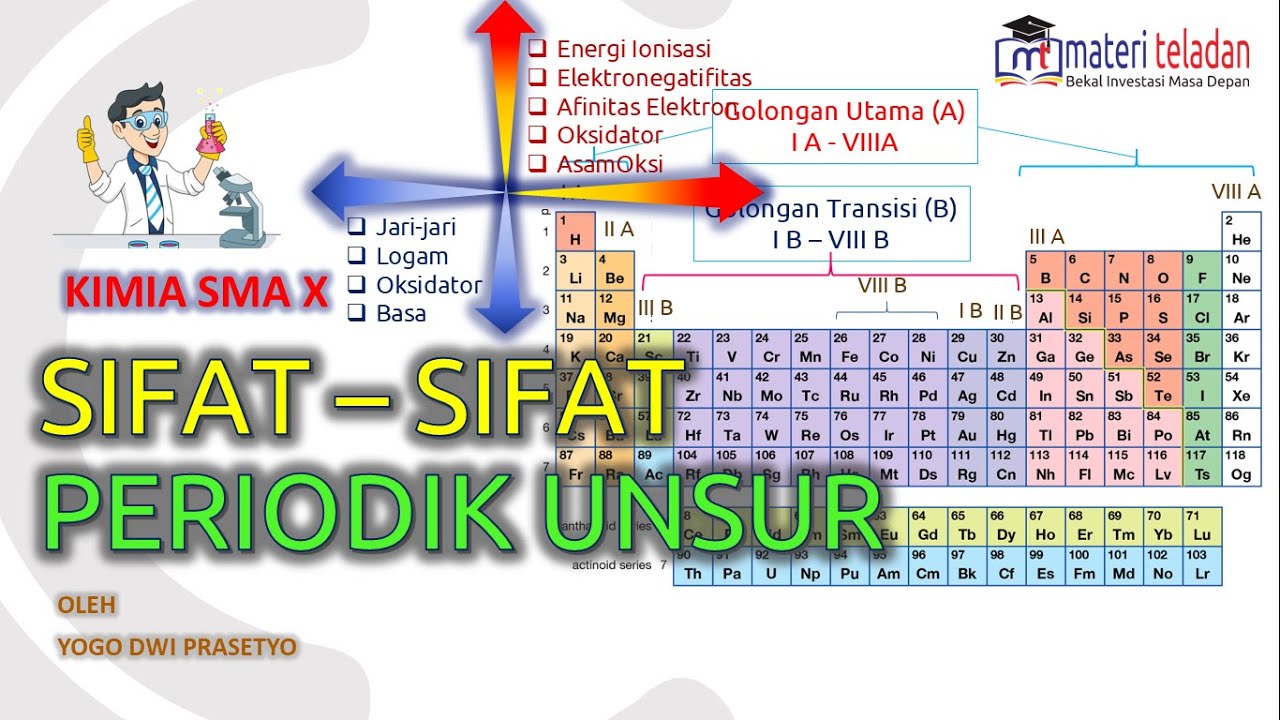
TLDRThis educational video explains the periodic properties of elements, including atomic radius, ionization energy, electron affinity, electronegativity, and their trends in the periodic table. The script covers key concepts and their behavior across periods and groups, with examples to help students understand how these properties vary. It also includes practical exercises to help reinforce the material, making it ideal for chemistry learners. The video highlights important trends such as increasing ionization energy across a period and decreasing atomic radius down a group, providing a comprehensive guide to mastering periodic trends in chemistry.
Takeaways
- 😀 Understanding periodic properties of elements is crucial for grasping chemical behavior.
- 😀 Atomic radius increases as we move from right to left across a period and from top to bottom in a group.
- 😀 Ionization energy is the energy required to remove an electron, which increases across a period and decreases down a group.
- 😀 Electron affinity measures the energy change when an atom gains an electron, generally increasing across a period and decreasing down a group.
- 😀 Electronegativity increases across a period and decreases down a group, with halogens having the highest values.
- 😀 Melting and boiling points generally increase until group 4A, then sharply decrease in group 8A (noble gases).
- 😀 Metals tend to lose electrons, while non-metals tend to gain electrons, influencing their properties.
- 😀 The trend of increasing or decreasing atomic properties (radius, ionization energy, etc.) can be understood using periodic tables.
- 😀 The group number helps predict the chemical properties and behavior of elements based on electron configuration.
- 😀 The strength of metallic character increases from right to left and from top to bottom on the periodic table.
- 😀 For practical use, understanding these periodic trends helps in predicting and explaining chemical reactions and element behaviors.
Q & A
What is the definition of atomic radius?
-Atomic radius is defined as the distance from the nucleus of an atom to the outermost stable orbital of the atom's electrons. It is usually measured in picometers (pm).
How does the number of electron shells affect the atomic radius?
-As the number of electron shells increases, the atomic radius becomes larger. This is because additional shells increase the distance between the nucleus and the outermost electrons.
What impact does nuclear charge have on atomic radius?
-Nuclear charge refers to the number of protons in the nucleus, and as it increases, the attractive force on the electrons also increases. This results in the electrons being pulled closer to the nucleus, thus reducing the atomic radius.
How does ionization energy change across periods and groups?
-Ionization energy generally increases across a period (from left to right) because atoms are more compact and the electrons are harder to remove. In contrast, ionization energy decreases down a group (from top to bottom) because the outer electrons are farther from the nucleus and more shielded by inner electrons.
What is electron affinity and how does it vary in the periodic table?
-Electron affinity is the energy released or absorbed when an atom gains an electron to form a negative ion. It generally increases across a period (from left to right) and decreases down a group (from top to bottom). However, Group 7A elements have the highest electron affinity, while Group 8A elements (noble gases) have very low or near-zero electron affinity.
What is electronegativity, and how does it change across periods and down groups?
-Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. It increases across a period (from left to right) and decreases down a group (from top to bottom). The halogens (Group 7A) have the highest electronegativity values.
How do melting and boiling points behave across periods and within groups?
-In a period, melting and boiling points typically increase until Group 4A, after which they sharply decrease by the time you reach Group 8A. In a group, the melting and boiling points of metals decrease as you move down the group, while nonmetals tend to show an increase in these points as you move down.
How does the periodic table explain the trend in metallic character?
-Metallic character increases as you move from right to left across a period and from top to bottom within a group. This is because metals tend to lose electrons easily, and elements on the left side of the periodic table have lower ionization energies, making them more metallic.
Which element in the given example (Na, Mg, Al, Si, P) has the largest atomic radius, and why?
-In the given example, sodium (Na) has the largest atomic radius. This is because it is the farthest to the left in the periodic table, and atomic radius increases as you move from right to left in a period.
How do you determine which element has the highest ionization energy among a group of elements?
-To determine the element with the highest ionization energy, look for the element furthest to the right in a period (since ionization energy increases from left to right) and the one closest to the top of a group (since ionization energy increases as you move up a group).
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Sifat Periodik Unsur | Jari jari Atom | Energi Ionisasi | Afinitas Elektron | Elektronegativitas

Sistem Periodik Unsur • Part 6: Sifat Keperiodikan Unsur

SISTEM PERIODIK UNSUR [Sifat Sifat Periodik Unsur]

PROPRIEDADES DA TABELA PERIÓDICA | Resumo de Química Enem. Professor Felipe Sobis
Sifat Keperiodikan Unsur Kelas X

KIMIA KELAS 10 | Sistem Periodik Unsur - Sifat Keperiodikan
5.0 / 5 (0 votes)