Bond Line Structures
Summary
TLDRIn this educational video, Victor introduces Bond Line Structures as a simplified method for representing organic molecules, contrasting them with the more detailed Lewis structures. He explains how to convert a Lewis structure to a Bond Line structure by focusing on carbon-carbon and carbon-heteroatom bonds, while explicitly showing hydrogens on heteroatoms. Victor emphasizes the importance of accurately depicting the connectivity of atoms to avoid mistakes in chemical analysis. He also provides dos and don'ts for drawing Bond Line structures, such as using neat zigzags and proper placement of substituents, to ensure clarity and avoid ambiguity. The video concludes with examples of converting molecules back to Lewis structures, highlighting the need to account for all hydrogens and electron pairs.
Takeaways
- 🧪 Bond Line Structures are an efficient way to represent organic molecules, saving time compared to Lewis structures.
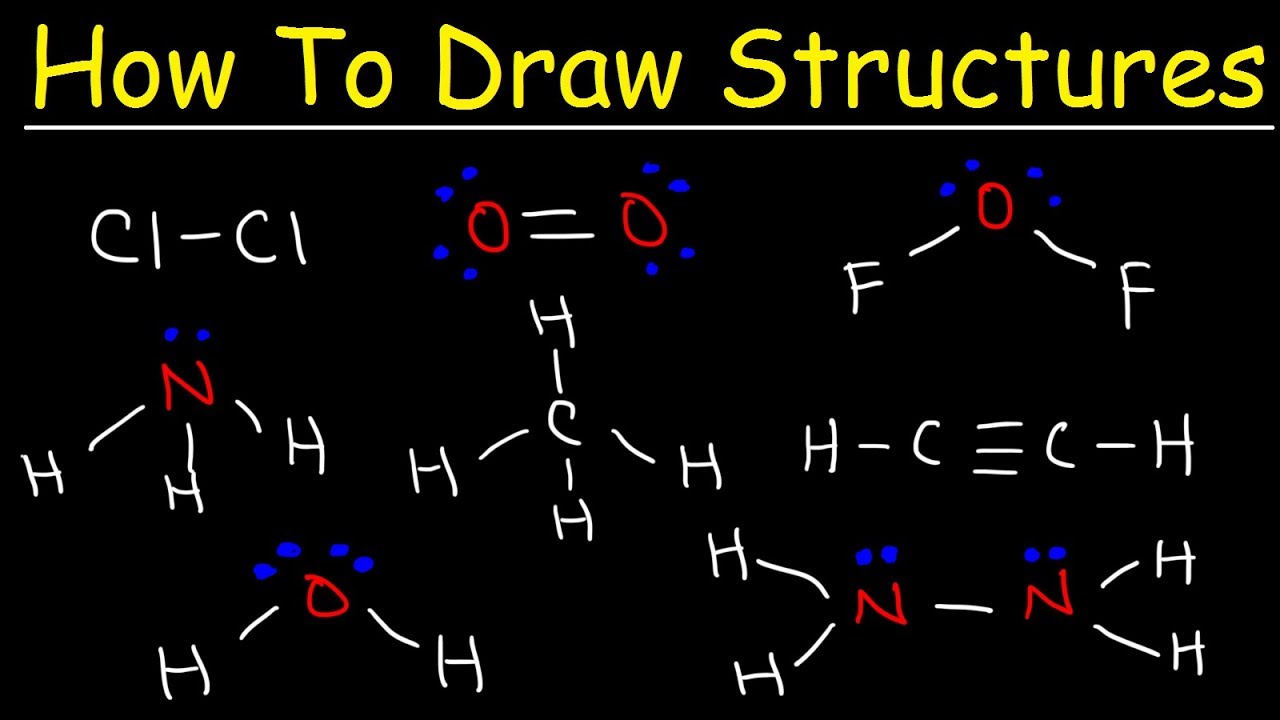
- 🔍 In Bond Line Structures, carbon-carbon bonds and bonds between carbons and heteroatoms are explicitly shown, while all other bonds are implicit.
- 📚 Heteroatoms like oxygen, nitrogen, sulfur, and phosphorus are not implicit in Bond Line Structures; they must be shown explicitly.
- ⚛️ Hydrogens on carbon atoms are implicit in Bond Line Structures, but hydrogens on heteroatoms must be shown.
- 🔄 The orientation of atoms in a Bond Line Structure can be altered for clarity without changing the molecule's identity, as long as connectivity is maintained.
- 🚫 It's crucial to avoid ambiguous or poorly drawn structures, as they can lead to misunderstandings and loss of points in assessments.
- 📏 Zigzag lines should be used when drawing Bond Line Structures to represent carbon chains, avoiding straight lines or exaggerated angles.
- ➡️ Substituents in a Bond Line Structure should be placed at proper locations, ensuring they are not ambiguously positioned.
- 🔬 Electron pairs can be shown in Bond Line Structures if necessary, such as for reactivity purposes, but are typically omitted unless required.
- 🔙 Converting from a Bond Line Structure to a Lewis structure involves counting carbons, adding missing hydrogens, and ensuring all atoms have the correct number of bonds.
Q & A
What is the main advantage of using Bond Line Structures over Lewis structures when drawing organic molecules?
-The main advantage of using Bond Line Structures is that they are less tedious and quicker to draw compared to Lewis structures, which show all atoms and bonds. This is particularly beneficial when dealing with larger molecules, as it saves time without losing the essential molecular information.
What are the key differences between Lewis structures, Condensed Lewis structures, and Bond Line structures?
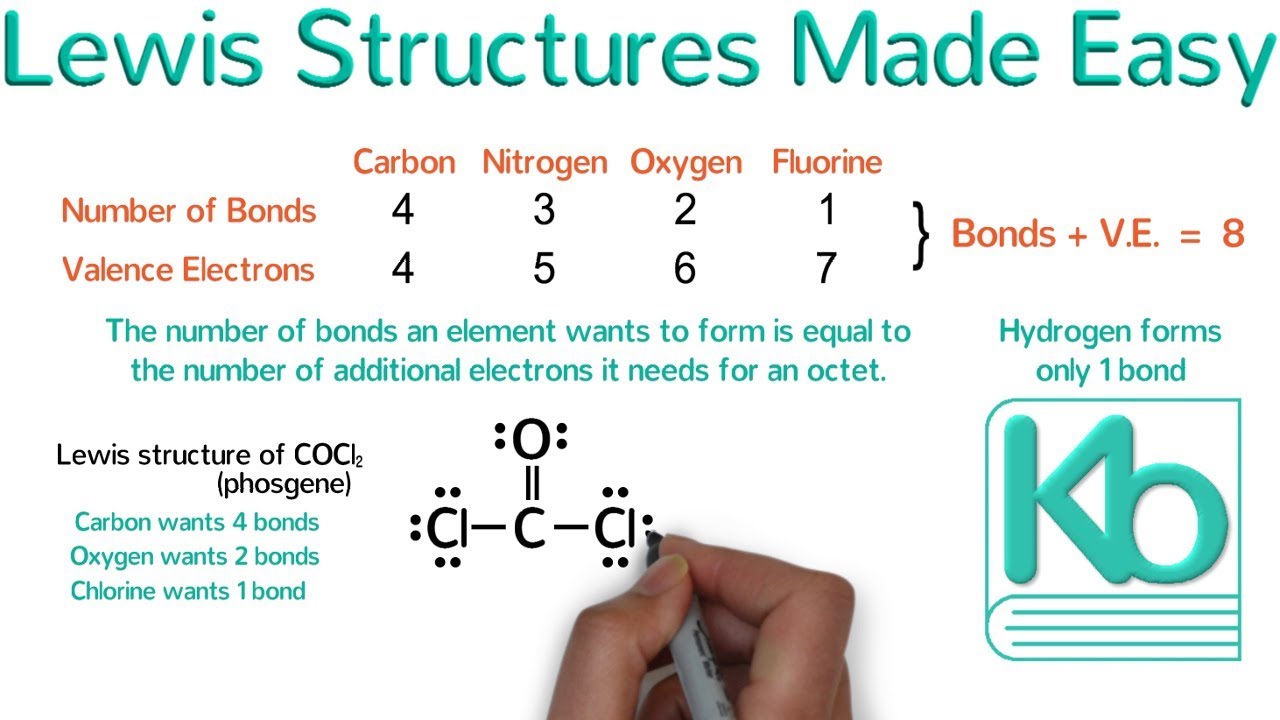
-Lewis structures show all atoms and bonds, Condensed Lewis structures do not show every single bond but still depict most atoms, while Bond Line structures focus on carbon-carbon and carbon-heteroatom bonds, omitting hydrogens on carbons but showing them on heteroatoms.
Why are heteroatoms not implicit in Bond Line structures?
-In Bond Line structures, heteroatoms are not implicit because they may have hydrogens or other substituents that need to be explicitly shown. This is in contrast to carbon atoms, where hydrogens are generally implicit unless they are part of a heteroatom.
What is the significance of showing hydrogens on heteroatoms in Bond Line structures?
-Showing hydrogens on heteroatoms in Bond Line structures is significant because it provides accurate information about the molecule's functional groups and potential reactivity, which is essential for understanding chemical behavior and reactions.
How does the orientation of atoms in a Bond Line structure affect the molecule's representation?
-The orientation of atoms in a Bond Line structure does not affect the molecule's representation as long as the connectivity remains the same. The structure can be drawn in a way that is aesthetically pleasing and clear, without altering the molecular identity.
Why is it important to avoid drawing Bond Line structures in a 'pike fence' manner?
-Drawing Bond Line structures in a 'pike fence' manner is discouraged because it can lead to ambiguity and misinterpretation of the molecule's structure. It is important to maintain a clear and consistent zigzag pattern to ensure the structure is easily readable and understood.
What is the role of electron pairs in Bond Line structures, and when are they typically shown?
-Electron pairs in Bond Line structures can be implicit, and they are typically shown when necessary for understanding reactivity or emphasizing specific molecular features. They are not always required unless they contribute to the molecule's function or reaction mechanisms.
How can one convert a Bond Line structure back to a Lewis structure?
-To convert a Bond Line structure back to a Lewis structure, one should count the carbon atoms and other heteroatoms, then add the necessary hydrogens to satisfy the valency of each carbon atom (four bonds per carbon). Electron pairs on heteroatoms should also be considered if they are part of the structure.
What are the 'dos and don'ts' of drawing Bond Line structures as mentioned in the script?
-When drawing Bond Line structures, one should 'do' neat zigzags and 'don't' make exaggerated angles or pike fences. 'Do' show substituents at proper locations and 'don't' draw ambiguous structures that could represent three-dimensional features without clear angles.
Why is it crucial not to forget implicit hydrogens and electron pairs when working with Bond Line structures?
-Forgetting implicit hydrogens and electron pairs when working with Bond Line structures can lead to incorrect interpretations of molecular properties and reactions. It is crucial to remember these elements to ensure accurate analysis and predictions of chemical behavior.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade Now5.0 / 5 (0 votes)