How To Draw Lewis Structures
Summary
TLDRThis video provides a comprehensive introduction to drawing simple Lewis structures. It explains how to determine the number of valence electrons in atoms like hydrogen, carbon, oxygen, and fluorine. The video also covers how atoms bond to form molecules, such as hydrogen gas (H2), chlorine gas (Cl2), methane (CH4), and water (H2O), while highlighting trends in bond formation based on valence electrons. The video also discusses exceptions like boron and beryllium and explores more complex molecules like hydrazine (N2H4) and acetylene (C2H2), helping viewers understand the process of drawing Lewis structures for various compounds.
Takeaways
- 😀 Hydrogen has one valence electron and forms a single bond in its Lewis structure.
- 😀 Beryllium, with two valence electrons, forms two bonds in its Lewis structure.
- 😀 Carbon has four valence electrons and typically forms four bonds to satisfy the octet rule.
- 😀 Nitrogen, with five valence electrons, forms three bonds to achieve a full octet.
- 😀 Oxygen has six valence electrons and commonly forms two bonds to complete its octet.
- 😀 Fluorine has seven valence electrons and prefers to form one bond to reach a stable octet.
- 😀 Boron, which has three valence electrons, typically forms only two bonds, not following the octet rule.
- 😀 Beryllium, with two valence electrons, also forms two bonds, differing from the octet rule.
- 😀 The number of bonds an atom forms is often determined by its position on the periodic table, with elements on the right typically acquiring electrons, and those on the left giving them away.
- 😀 In Lewis structures, the number of valence electrons corresponds to the number of bonds an atom is likely to form.
- 😀 Molecules like oxygen (O2) and methane (CH4) demonstrate how atoms with specific valence electrons combine to form stable structures, with oxygen forming double bonds and carbon forming four single bonds in methane.
Q & A
What is the first step in drawing a Lewis structure?
-The first step in drawing a Lewis structure is to determine the number of valence electrons in the atom. Valence electrons are the outermost electrons in an atom, and they are crucial for bonding.
How many valence electrons does hydrogen have, and what does this mean for its Lewis structure?
-Hydrogen has one valence electron, and when drawing its Lewis structure, it is represented by a single dot. Hydrogen can only form one bond to satisfy the octet rule, meaning it pairs with another hydrogen atom in the case of H2.
What is the typical bonding behavior of halogens like chlorine and fluorine?
-Halogens like chlorine and fluorine have seven valence electrons and prefer to form only one bond, as they only need one additional electron to complete their octet.
What is the octet rule, and how does it relate to the number of bonds an atom forms?
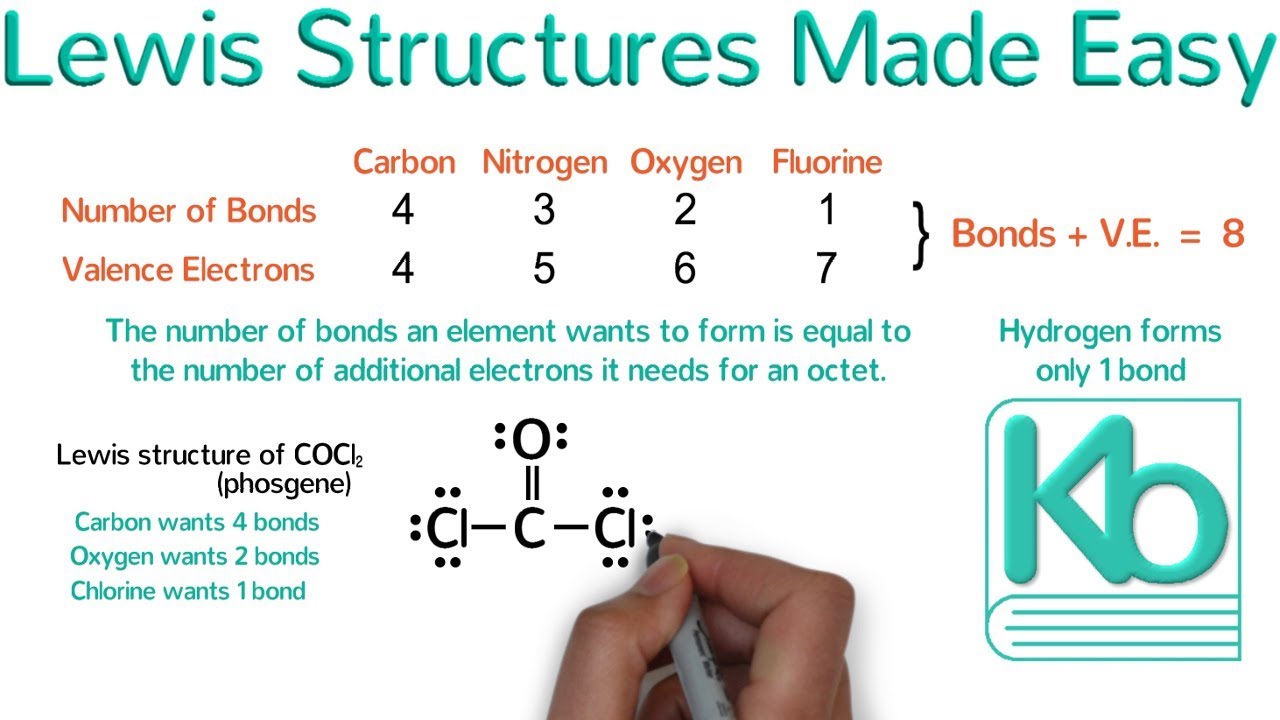
-The octet rule states that atoms tend to form bonds in such a way that they have eight electrons in their valence shell, resembling the electron configuration of a noble gas. This is why elements like carbon form four bonds, nitrogen three, oxygen two, and fluorine one.
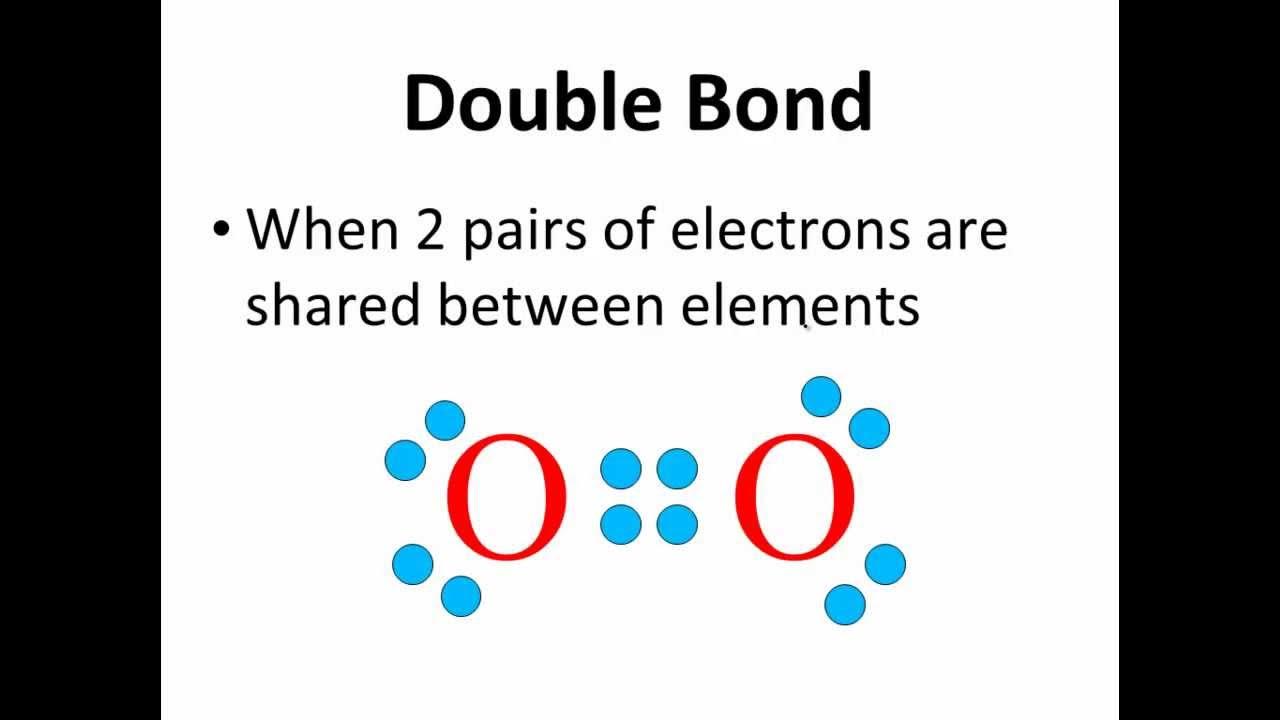
Why does oxygen form a double bond in its Lewis structure, and how does this impact its electron configuration?
-Oxygen has six valence electrons and needs two more electrons to complete its octet. Therefore, it forms a double bond with another oxygen atom, using four electrons to satisfy the octet rule.
What are the common bonding preferences of elements on the right side of the periodic table, such as carbon, nitrogen, and oxygen?
-Elements on the right side of the periodic table, such as carbon, nitrogen, and oxygen, typically acquire electrons to complete their octets. Carbon forms four bonds, nitrogen three, and oxygen two bonds to reach eight valence electrons.
How does the Lewis structure of methane (CH4) illustrate the bonding preferences of carbon and hydrogen?
-In methane, carbon forms four single bonds with four hydrogen atoms. This satisfies carbon's need for four bonds (to reach eight valence electrons) and hydrogen's need for one bond (to reach two valence electrons).
Why does nitrogen form three bonds in ammonia (NH3), and how is this reflected in its Lewis structure?
-Nitrogen has five valence electrons and needs three more to complete its octet. In ammonia, nitrogen forms three single bonds with three hydrogen atoms, which satisfies its bonding needs and follows the rule for its preferred number of bonds.
How is the bent shape of water (H2O) reflected in its Lewis structure, and why does this happen?
-Water has a bent molecular shape due to the repulsion between lone pairs of electrons on the oxygen atom. In the Lewis structure, oxygen forms two single bonds with hydrogen, but the lone pairs push the bonds into a bent shape rather than a linear one.
What is the Lewis structure for acetylene (C2H2), and why is it considered the best structure for the molecule?
-Acetylene (C2H2) has a triple bond between the two carbon atoms, with each carbon also forming a single bond with a hydrogen atom. This structure satisfies the bonding preferences of both carbon (which needs four bonds) and hydrogen (which needs one bond). The triple bond is the best configuration to complete the carbon's octet while maintaining proper bonding with hydrogen.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Bond Line Structures

Ikatan Kimia • Part 3: Ikatan Kovalen, Struktur Lewis, Aturan Oktet

How to Draw Lewis Structures: Five Easy Steps
Lewis Structures Made Easy: Examples and Tricks for Drawing Lewis Dot Diagrams of Molecules
Lewis Dot Structures for Covalent Compounds - Part 1 CLEAR & SIMPLE

Getting Started with SketchUp Pro 2025! (BEGINNERS START HERE!)
5.0 / 5 (0 votes)