PRAKTIKUM GEOKIMIA-Teknik Preparasi Sampel AAS
Summary
TLDRThis video tutorial from the Geochemistry Practice AAS course demonstrates the complete process of sample preparation for Atomic Absorption Spectroscopy (AAS). It covers two main methods: dry destruction for organic samples like leaves, involving oven and furnace ashing followed by acid dissolution, and wet destruction for inorganic samples like rocks, involving crushing, treatment with aquaregia, heating, and filtration. The tutorial clearly outlines required tools, chemicals, and step-by-step procedures, ensuring accurate preparation of solutions ready for AAS analysis. The session is designed to guide viewers through precise laboratory techniques for reliable analytical results.
Takeaways
- 😀 Dry destruction starts with weighing 1 gram of an organic sample, such as a leaf, and placing it in a porcelain dish for heating.
- 😀 Heat the organic sample in an oven at 105-110°C for 30 minutes before moving it to a furnace for 8 hours at 450°C until completely dry.
- 😀 After ashing, add 2 ml of 10 M HCl to the sample and heat it on a hotplate at 60°C for 30 minutes with 200 RPM.
- 😀 Transfer the dissolved ash into a 50 ml volumetric flask, then dilute it with 0.1 M HNO3 until the calibration mark is reached for analysis.
- 😀 Wet destruction begins with weighing 1 gram of an inorganic sample, such as a rock, and crushing it into powder using a mortar and pestle.
- 😀 The powdered rock sample is transferred into a 100 ml beaker, and 3 ml of aqua regia (HNO3 + HCl) is added.
- 😀 Heat the rock sample with aqua regia on a hotplate at 60°C for 30 minutes with 200 RPM until the sample completely dissolves.
- 😀 After dissolution, filter the solution using filtering paper to remove any remaining impurities.
- 😀 The filtered solution is then transferred into a sample bottle, making it ready for analysis.
- 😀 Both dry and wet destruction methods are used to prepare samples for Atomic Adsorption Spectroscopy (AAS) analysis.
- 😀 The proper use of equipment like measuring cylinders, funnels, and volumetric flasks is crucial for accurate sample preparation.
Q & A
What are the two types of sample preparations discussed in the AAS procedure?
-The two types of sample preparations are dry destruction and wet destruction.
What materials are required for the AAS sample preparation process?
-The materials required include a measuring cylinder, watch glass, volumetric flask (50 ml), funnel, evaporating porcelain dish, pipette, sample bottle, mortar and pestle, filtering paper, hotplate, HNO3 0.1 M, aquaregia, HCl 10 M, leaf sample, and rock sample.
How is the leaf sample prepared in the dry destruction process?
-The leaf sample is weighed (1 gram), placed into a porcelain dish, heated in an oven at 105-110°C for 30 minutes, then transferred to a furnace at 450°C for 8 hours until it is completely ashed.
What happens after the leaf sample is ashed in the dry destruction method?
-After ashing, 2 ml of HCl 10 M is added to the sample, which is then heated on a hotplate at 60°C for 30 minutes to dissolve the ash. The solution is then transferred to a volumetric flask and diluted with HNO3 0.1 M to the calibration mark.
How is the rock sample prepared in the wet destruction process?
-The rock sample is crushed into powder using a mortar and pestle, weighed (1 gram), and placed into a 100 ml beaker. 3 ml of aquaregia is added, and the sample is heated on a hotplate at 60°C for 30 minutes.
What is aquaregia and how is it used in the wet destruction process?
-Aquaregia is a mixture of one part HNO3 (nitric acid) and three parts HCl (hydrochloric acid). In the wet destruction process, 3 ml of aquaregia is added to the rock sample to help dissolve it.
What happens after the rock sample dissolves in the wet destruction process?
-Once the rock sample dissolves completely, the solution is filtered using filtering paper, and the filtered solution is transferred to a sample bottle for analysis.
What is the purpose of heating the sample during both the dry and wet destruction processes?
-Heating the sample helps to break down the sample materials and facilitate the dissolution of substances in both the dry and wet destruction processes.
Why is it important to heat the dry sample at 450°C for 8 hours?
-Heating the sample at 450°C for 8 hours ensures that the organic material in the sample is completely ashed, leaving behind only inorganic components that can be further processed.
What is the final step in both sample preparation processes?
-The final step in both processes is transferring the dissolved sample to a volumetric flask and diluting it to the calibration mark with the appropriate solution (HNO3 0.1 M for dry destruction, and the filtered solution for wet destruction). The sample is then ready for analysis.
Outlines

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифMindmap

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифKeywords

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифHighlights

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифTranscripts

Этот раздел доступен только подписчикам платных тарифов. Пожалуйста, перейдите на платный тариф для доступа.
Перейти на платный тарифПосмотреть больше похожих видео

Metode pengujian logam Fe dengan AAS

Determination of Zinc by Linear Calibration and Standard Addition Methods using AAS
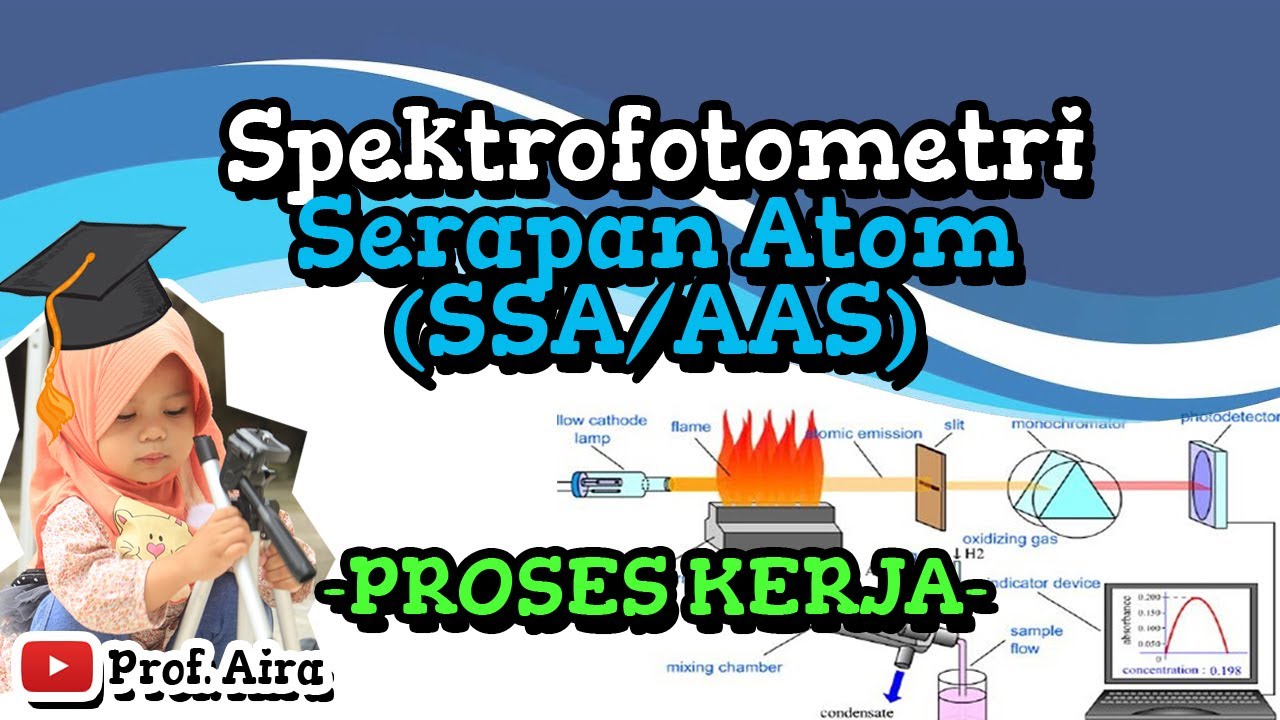
SPEKTROFOTOMETRI SERAPAN ATON (SSA/AAS) – PROSES KERJA

Part 1 : Analisa Cu : Air Minum Dalam Kemasan (SNI 3553:2015) dengan Instrumen AAS Shimadzu AA7000

Quickly Understand Atomic Absorption Spectroscopy (AAS)
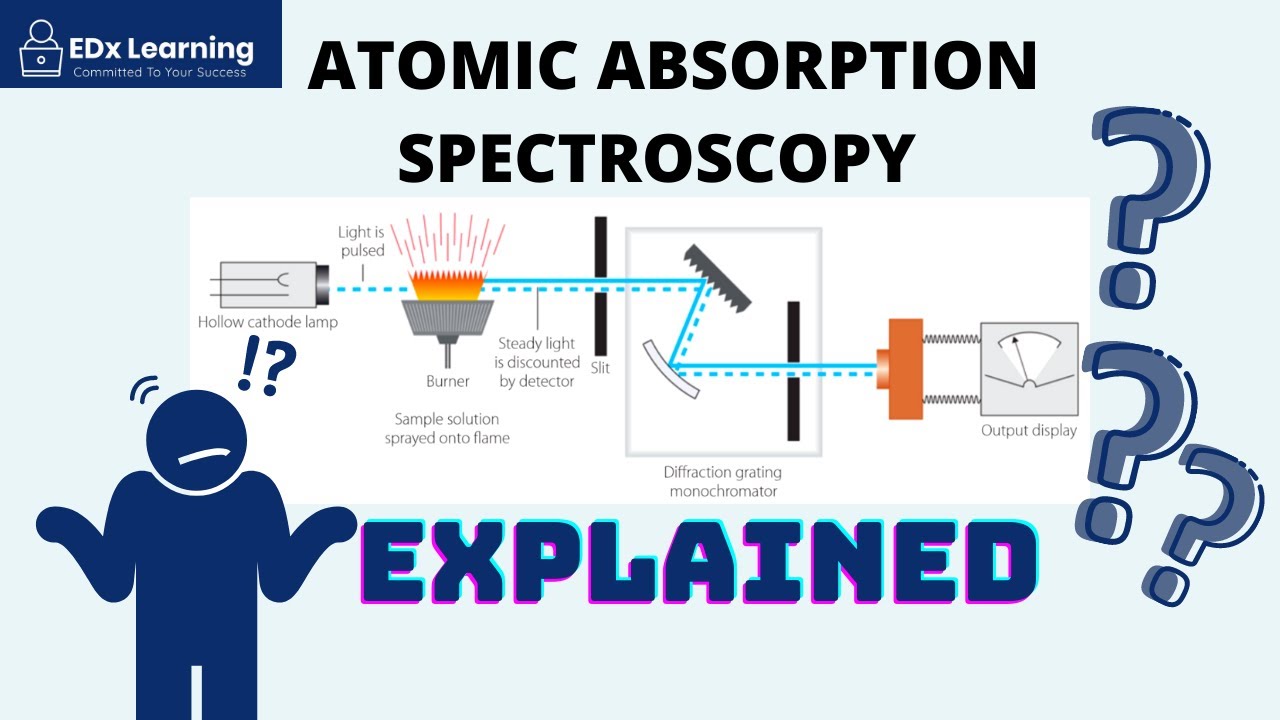
Atomic Absorption Spectroscopy (AAS) Explained - PART 1
5.0 / 5 (0 votes)
