Metode pengujian logam Fe dengan AAS
Summary
TLDRThis video explains the process of testing for iron (Fe) levels in clean water using Atomic Absorption Spectroscopy (AAS). The procedure includes preparing samples with concentrated nitric acid, creating calibration solutions, and optimizing the AAS instrument. It also emphasizes the importance of accuracy in determining iron concentrations, as this directly affects water quality. The video walks through each step, from sample preparation to calibration curve creation and final measurement, ensuring reliable results. Understanding these procedures helps ensure clean water meets safety standards.
Takeaways
- 😀 The video introduces the process of testing the iron (Fe) content in clean water using Atomic Absorption Spectrophotometry (AAS).
- 😀 The key equipment used in this test includes AAS, acetylene gas, and various glassware like beakers, flasks, and pipettes.
- 😀 To prepare the sample, 100 ml of water is taken and treated with concentrated nitric acid (HNO3) to dissolve the iron content.
- 😀 The sample is heated until the volume is reduced to about 10-20 ml, and further acid is added if the sample is not clear.
- 😀 After the sample is clear, it's filtered, transferred to a volumetric flask, and diluted with mineral-free water.
- 😀 Calibration solutions are prepared with specific concentrations (100 ppm, 10 ppm) using a standard iron solution.
- 😀 A calibration curve is created by measuring absorbance at specific wavelengths to ensure accurate results.
- 😀 The AAS instrument is optimized, and absorbance is measured for both blank and working solutions.
- 😀 A good calibration curve has a linear correlation coefficient (R) of at least 0.995 to ensure reliable results.
- 😀 The concentration of iron in the water sample is determined by measuring its absorbance and comparing it with the calibration curve.
- 😀 The test helps ensure the safety of drinking water by detecting if the iron content is within acceptable levels for consumption.
Q & A
What is the primary goal of this procedure?
-The primary goal of this procedure is to test the concentration of dissolved iron (Fe) in clean water using Atomic Absorption Spectrophotometry (AAS).
What are the essential chemicals required for the test?
-The essential chemicals required for the test are concentrated nitric acid and a stock solution of iron (Fe) at 1000 PPM.
What is the role of nitric acid in the sample preparation?
-Nitric acid is used to break down the sample and ensure that the iron is fully dissolved in the solution, making it suitable for analysis using AAS.
What equipment is necessary for conducting the AAS test?
-The necessary equipment includes an atomic absorption spectrophotometer, a burner, hollow cathode lamps for iron, volumetric flasks, pipettes, and various glassware like funnels and beakers.
How do you prepare the sample for testing?
-The sample is homogenized, and 100 mL is measured and placed into a beaker. Nitric acid is added, and the mixture is heated until the volume reduces to 10-20 mL. This process is repeated until the sample becomes clear or slightly white.
What is the purpose of creating a calibration curve?
-The calibration curve is created to correlate the absorbance readings with known concentrations of iron. It is essential for determining the concentration of iron in the test samples accurately.
What steps are involved in preparing the standard iron solutions?
-To prepare standard solutions, 10 mL of the 1000 PPM stock solution is diluted to 100 mL to make a 100 PPM solution. Then, a 10 mL aliquot of the 100 PPM solution is diluted to 100 mL to make a 10 PPM solution.
What should be done if the correlation coefficient (R) of the calibration curve is below 0.995?
-If the correlation coefficient (R) is less than 0.995, the instrument should be checked, and the calibration procedure should be repeated to obtain a better result.
Why is it important to use water free of minerals during the sample preparation?
-Mineral-free water is used to avoid introducing additional contaminants or interference that could affect the accuracy of the test results.
What should be done if the absorption reading of a sample exceeds the optimal range?
-If the absorption reading exceeds the optimal range, the sample should be diluted and measured again to bring the reading within the acceptable range for analysis.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Analisis Kadar Logam Besi (Fe) Pada Air Minum Dalam Kemasan Menggunakan Metode SSA
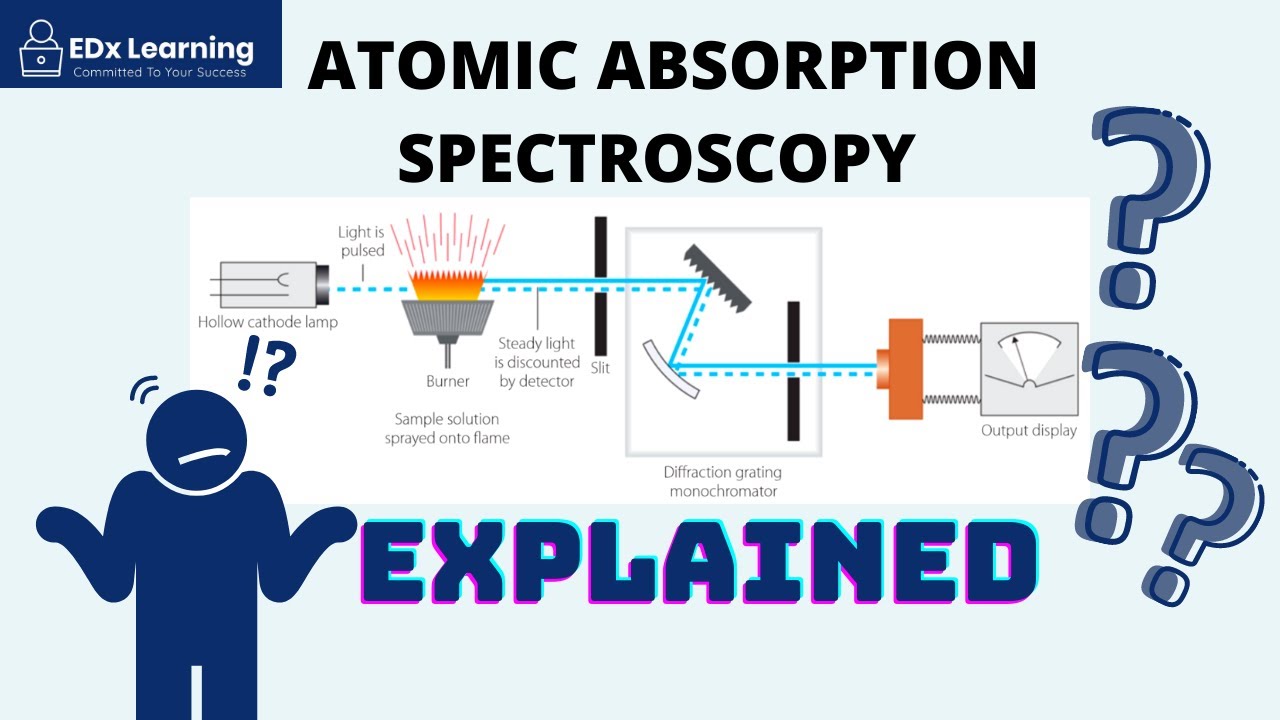
Atomic Absorption Spectroscopy (AAS) Explained - PART 1

analisis kandungan logam kadmium pada daging dengan metode AAS

Quickly Understand Atomic Absorption Spectroscopy (AAS)
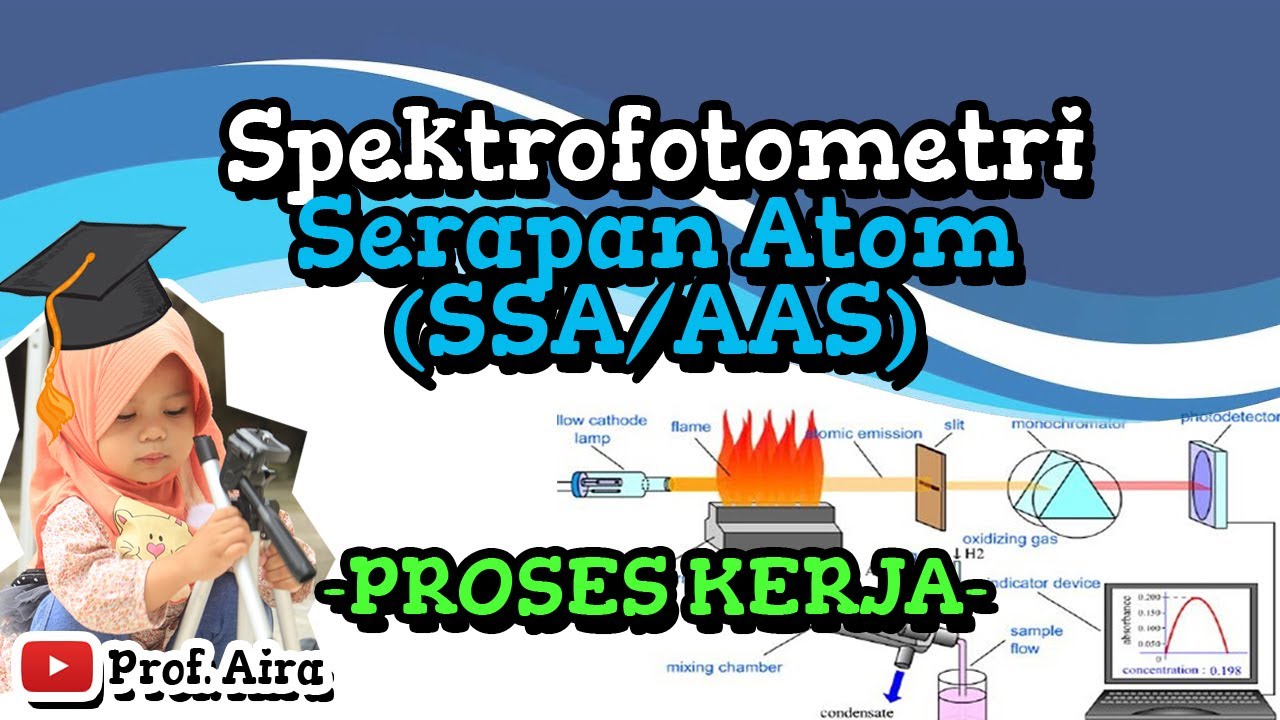
SPEKTROFOTOMETRI SERAPAN ATON (SSA/AAS) – PROSES KERJA

Determination of Zinc by Linear Calibration and Standard Addition Methods using AAS
5.0 / 5 (0 votes)