Atomic Absorption Spectrometry (AAS)
Summary
TLDRThis video demonstrates the process of determining trace levels of zinc in food samples using flame atomic absorption spectrometry (FAAS). The procedure involves preparing zinc standards with varying concentrations, digesting a food sample with hydrochloric acid, filtering and purifying the solution, and performing a percent recovery test with spiked and unspiked solutions. The absorbance of the standards, food sample, and both spiked and unspiked samples are then measured using FAAS to accurately quantify zinc levels. This method ensures precise analysis and validation through multiple preparation and purification steps.
Takeaways
- 😀 Zinc standards are prepared by creating a series of standard solutions with concentrations from 0.0 to 1 mg per liter.
- 😀 Precise volumes of the stock solution are transferred into 25 mL volumetric flasks, followed by adding hydrochloric acid and dilution with deionized water.
- 😀 These standard solutions are used to calibrate the flame atomic absorption spectrometer (FAS).
- 😀 A 2.0 g food sample is weighed and placed into a 100 mL conical flask for digestion.
- 😀 12.0 mL of 6.0 M hydrochloric acid is added to the food sample, and it is boiled for 15 minutes on a hot plate inside a fume hood.
- 😀 The sample becomes viscous and dark brown during the digestion process.
- 😀 After cooling, the sample is diluted with deionized water, filtered, and transferred into a 50 mL volumetric flask for further dilution.
- 😀 The sample is then centrifuged for 5 to 10 minutes and filtered through a 0.45 micron filter for further purification.
- 😀 A percent recovery test is performed using spiked and unspiked solutions to ensure accuracy in measurements.
- 😀 The absorbance of the prepared standards, food sample solution, and both spiked and unspiked samples are measured using flame atomic absorption spectrometry (FAS).
Q & A
What is the primary objective of this experiment?
-The primary objective of the experiment is to determine trace levels of zinc in food samples using flame atomic absorption spectrometry (FAS).
How are the zinc standards prepared for this experiment?
-Zinc standards are prepared by creating a series of standard solutions with concentrations ranging from 0.0 to 1 mg per liter. Precise volumes of the stock solution are transferred into 25 ml volumetric flasks, and 3 ml of 6.0 molarity hydrochloric acid is added before diluting to the 25 ml mark with deionized water.
What is the purpose of adding hydrochloric acid to the zinc standards?
-Hydrochloric acid is added to the zinc standards to help dissolve the zinc and ensure it is in a suitable form for measurement by the flame atomic absorption spectrometer.
What is the next step after preparing the zinc standards?
-After preparing the zinc standards, the next step is to prepare the food sample by weighing out exactly 2.0 g of the sample and placing it into a 100 ml conical flask.
How is the food sample treated for digestion?
-The food sample is treated by adding 12.0 ml of 6.0 molarity hydrochloric acid and bringing the mixture to a slow boil on a hot plate for 15 minutes. This digestion process turns the sample viscous and dark brown.
What should be done after the digestion of the food sample?
-Once the sample has cooled to room temperature, approximately 30 ml of deionized water is added, and the mixture is mixed well before being filtered using vacuum filtration. The clear filtrate is transferred into a 50 ml volumetric flask and topped up to the mark with deionized water.
Why is centrifugation performed in this experiment?
-Centrifugation is performed to further purify the food sample solution by separating solid particles from the liquid. This helps ensure the sample is clear for accurate analysis.
What is the role of the 0.45 micron filter in this experiment?
-The 0.45 micron filter is used to filter approximately 20 ml of the solution, removing any remaining impurities or particles that could interfere with the analysis.
What is the purpose of performing a percent recovery test?
-The percent recovery test is performed to ensure the accuracy of the analysis. It involves comparing the results of unspiked and spiked food sample solutions to verify that the procedure correctly quantifies zinc levels.
How are the absorbances of the samples measured?
-The absorbances of the prepared standards, the food sample solution, and the spiked and unspiked samples are measured using flame atomic absorption spectrometry (FAS), which detects the concentration of zinc in the solutions.
Outlines

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードMindmap

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードKeywords

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードHighlights

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレードTranscripts

このセクションは有料ユーザー限定です。 アクセスするには、アップグレードをお願いします。
今すぐアップグレード関連動画をさらに表示

Lead Detection Using Flame AA Spectroscopy

ANALISIS CEMARAN LOGAM Pb, Cd, Zn DALAM MAKANAN DAN MINUMAN KEMASAN KALENG MENGGUNAKAN AAS/SSA

Penentuan Krom dalam Air Limbah Menggunakan AAS

Metode pengujian logam Fe dengan AAS
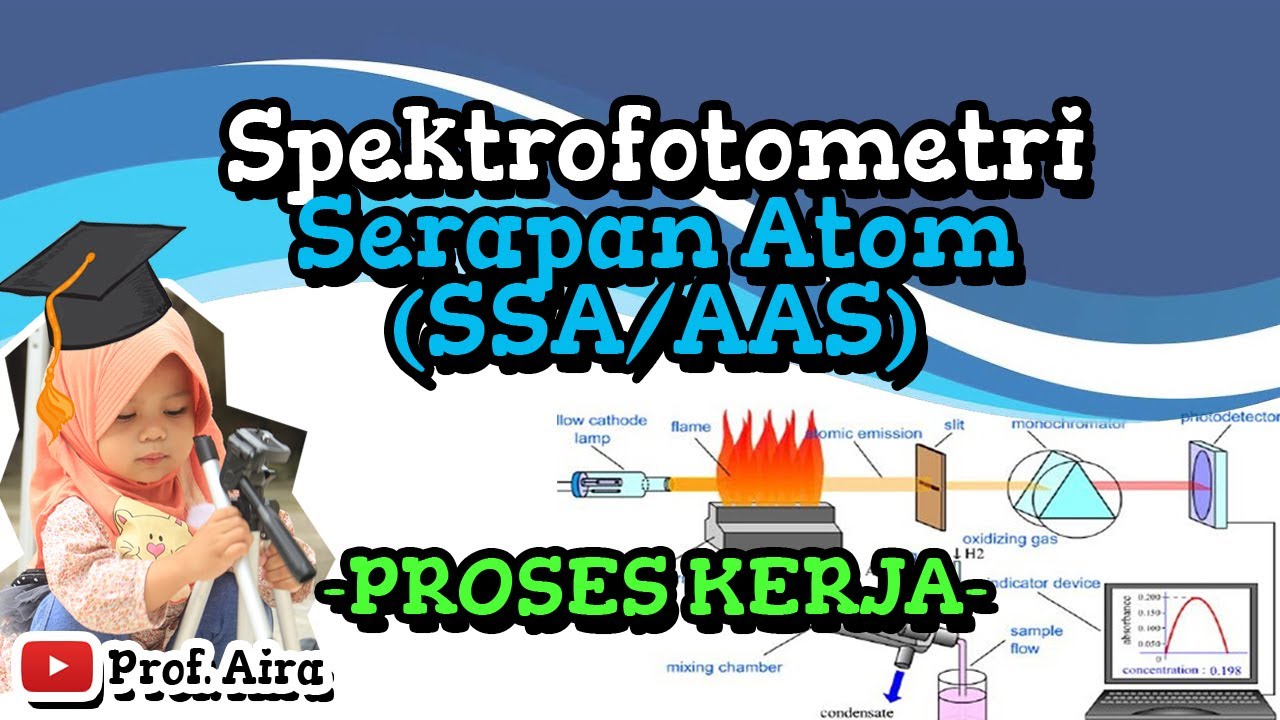
SPEKTROFOTOMETRI SERAPAN ATON (SSA/AAS) – PROSES KERJA
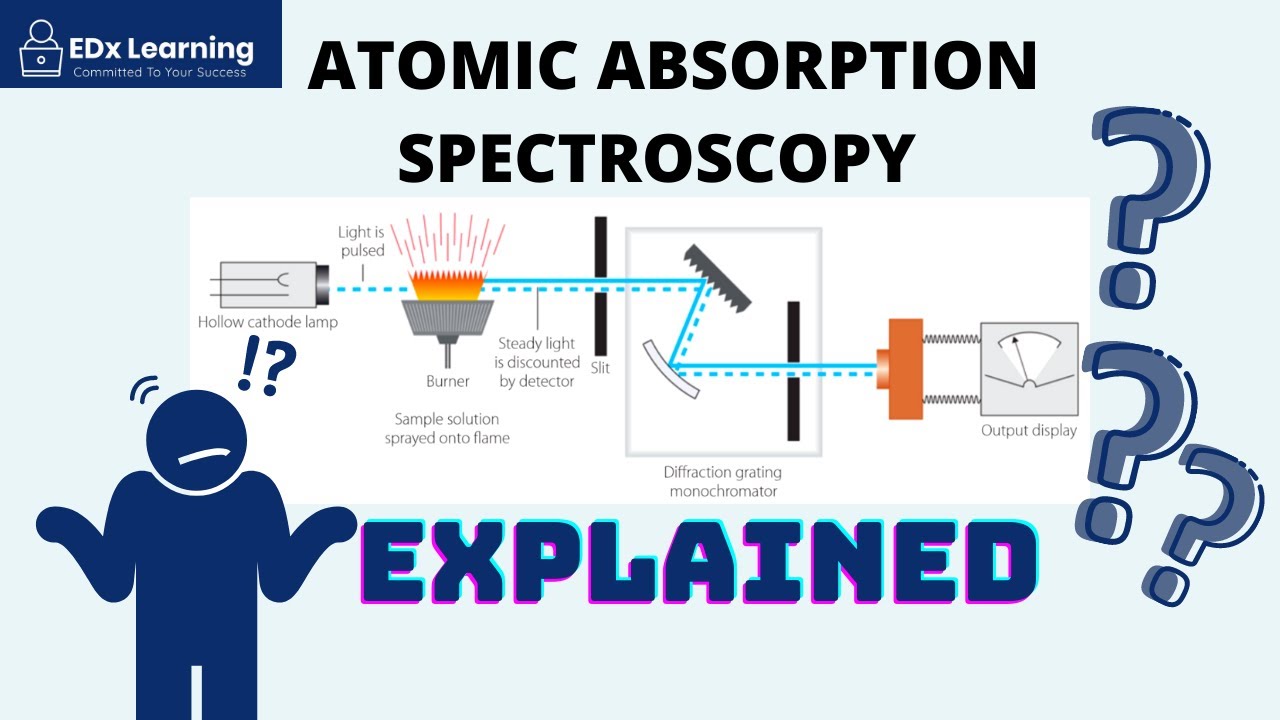
Atomic Absorption Spectroscopy (AAS) Explained - PART 1
5.0 / 5 (0 votes)
