Lead Detection Using Flame AA Spectroscopy
Summary
TLDRThe video demonstrates the operation of a flame atomic absorption spectrometer, focusing on lead analysis. It showcases the setup, including the gas tank, sample introduction, and various light sources. The narrator explains the process of preparing the instrument, calibrating it with standards, and running samples. As samples are introduced, the color changes in the flame reveal the presence of lead. Calibration curves are established to correlate concentration with absorbance. Overall, the video emphasizes the precision and technical aspects of using this instrument for metal analysis, making it educational and engaging for viewers.
Takeaways
- 🔥 The flame atomic absorption (AA) spectrometer is equipped with essential components, including a gas tank, acetylene line, and computer for data capture.
- 💡 Each metal analysis requires specific light sources; the lead lamp is activated for lead detection.
- 💧 Samples are introduced into the flame via a water bottle and sip tube, allowing for efficient vaporization.
- 🔍 The light emitted from the excited sample is collected by a detector, which transmits data to the computer for analysis.
- 📏 Calibration is crucial, involving a comparison of concentration (mg/L) against absorbance (unitless) to ensure accurate results.
- 🧪 The analysis involves careful handling of solutions to avoid contamination, including wiping the sample containers.
- 🌈 Observing the flame color changes helps indicate the presence and concentration of lead in the samples.
- 📊 The calibration curve is established to correlate absorbance levels with known concentrations, aiding in precise measurements.
- 👩🔬 Collaborative learning is emphasized, as students work together to operate the instrument and analyze samples.
- 🤝 The educational environment encourages hands-on experience with scientific instruments, enhancing practical understanding of analytical chemistry.
Q & A
What is the primary purpose of the setup described in the transcript?
-The setup is designed for analyzing metal concentrations, specifically lead, using a flame emission spectrometry technique.
What components are involved in the flame analysis process?
-The main components include a gas tank, acetylene supply, light sources, a sample introduction system, and a detector that captures emitted light data.
How does the sample introduction work in this setup?
-The sample is introduced into the flame via a water bottle and a sip tube, where it is vaporized before analysis.
What happens to the light emitted from the sample?
-The emitted light is collected by the detector, which then sends the data to a computer for analysis and numerical output.
What is the significance of calibrating the instrument before running samples?
-Calibration ensures accurate measurements by establishing a relationship between the concentration of the metal and the absorbance reading.
What does the calibration curve represent?
-The calibration curve represents the relationship between concentration (in milligrams per liter) and absorbance (a unitless measure) for the analysis.
What should be done with the solutions before they are analyzed?
-The solutions should be wiped down to remove any contaminants before being inserted into the instrument for analysis.
How does the flame's color indicate the presence of lead in the sample?
-The color change of the flame to orange indicates a high concentration of lead, which can be visually observed during the analysis.
What role does the DI (deionized) water play in the process?
-DI water is used to clean the sample container and may also be used to blank out the instrument before running samples to ensure accuracy.
Who are the individuals mentioned in the transcript, and what is their role in the process?
-Wesley Sutra is a participant in the class conducting the analysis, and there are references to other students in the class involved in handling the instrument and solutions.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

Part 1 : Analisa Cu : Air Minum Dalam Kemasan (SNI 3553:2015) dengan Instrumen AAS Shimadzu AA7000
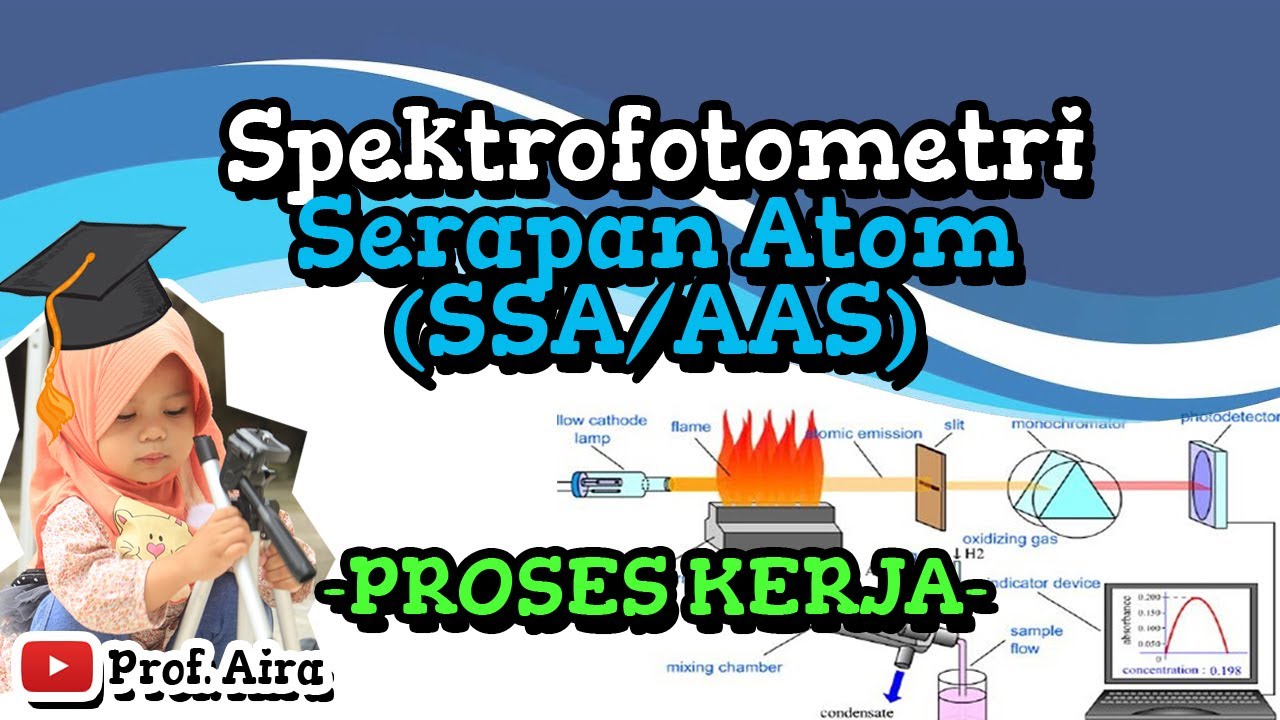
SPEKTROFOTOMETRI SERAPAN ATON (SSA/AAS) – PROSES KERJA
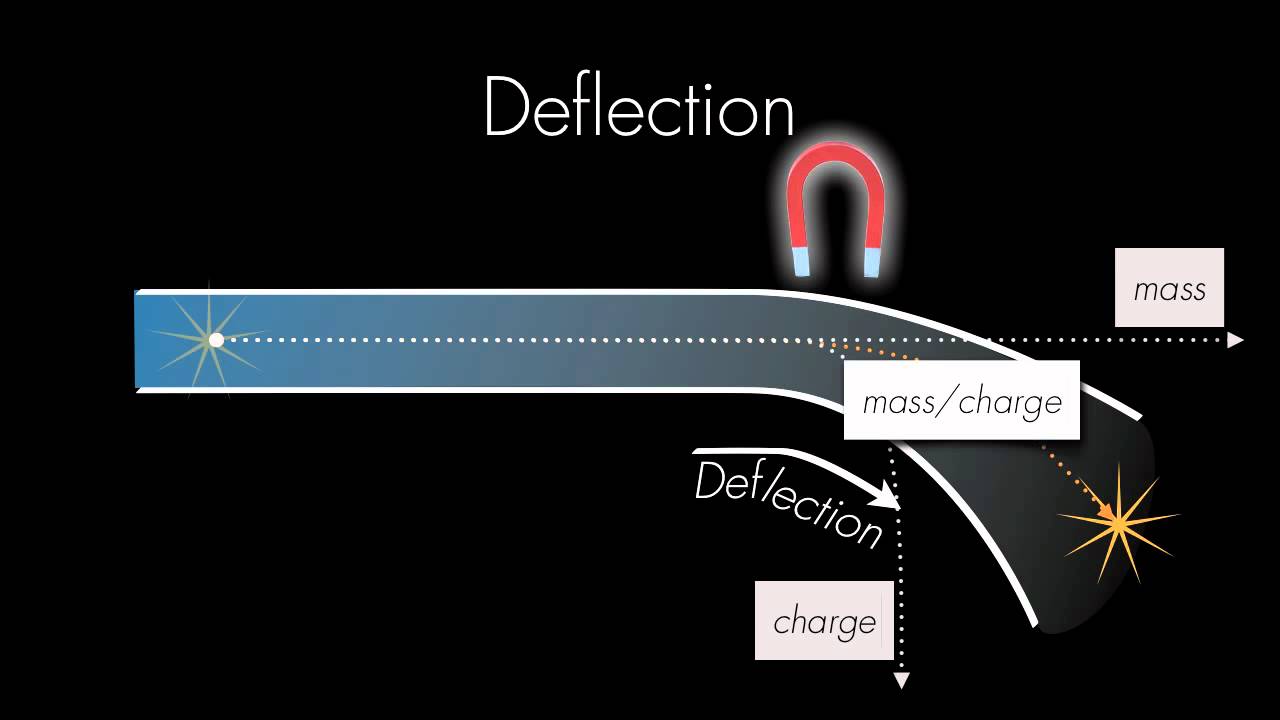
2.2 The Mass Spectrometer

Atomic Absorption Spectrometry (AAS)

Lecture 60: Sampling and Analysis of PM10 & PM2.5 using Spectrometer
Isotopes and Mass Spectrums
5.0 / 5 (0 votes)