KALORIMETER : Menghitung Perubahan Entalpi dengan Kalorimetri - Kimia kelas XI
Summary
TLDRIn this video, the presenter explains how to determine enthalpy changes using a calorimeter. The discussion focuses on two types of calorimeters: a bomb calorimeter used for combustion reactions and a simple calorimeter for mixing reactions. The video includes detailed calculations and examples, such as the combustion of methane and neutralization reactions between NaOH and HCl, using formulas to determine heat changes. The presenter also covers essential terms like specific heat capacity and provides step-by-step guidance for solving calorimetry problems, aimed at helping students understand thermochemical calculations in practical scenarios.
Takeaways
- 😀 A calorimeter is a device used to measure heat changes in a chemical reaction, specifically heat released or absorbed during the process.
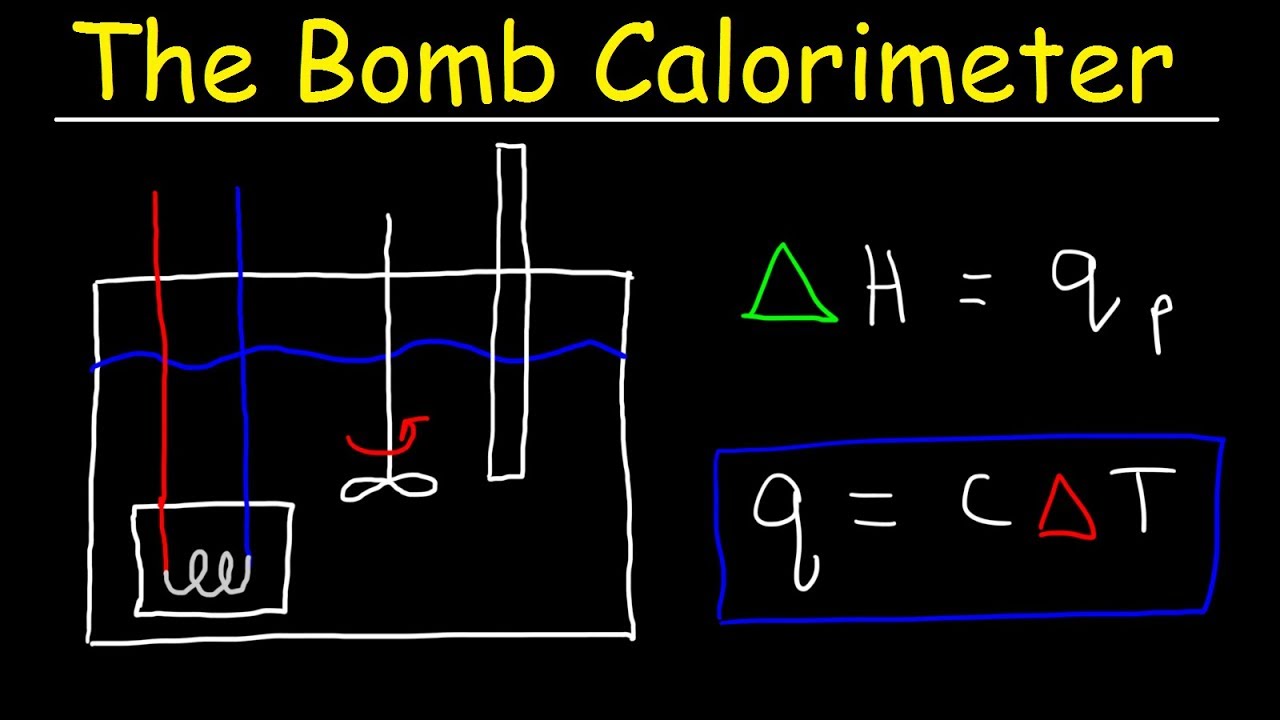
- 😀 Bomb calorimeters are used for combustion reactions, where the sample is placed in a sealed container and the heat released is absorbed by the surrounding water.
- 😀 Simple calorimeters use materials like styrofoam and are often used for simpler reactions, such as mixing two solutions or dissolving a substance in water.
- 😀 The heat absorbed by water in a calorimeter can be calculated using the formula Q = mcΔT, where m is the mass, c is the specific heat capacity, and ΔT is the temperature change.
- 😀 To calculate the enthalpy change (ΔH) of a reaction, we can divide the total heat absorbed by the reaction by the number of moles of the substance involved.
- 😀 A key concept in thermochemistry is that the heat released by the system (reaction) is absorbed by the surroundings (water and calorimeter), which is why their heat is calculated together.
- 😀 In bomb calorimeter calculations, the total heat is the sum of the heat absorbed by both the water and the calorimeter itself.
- 😀 To convert heat into a value per mole (kJ/mol), divide the total heat by the number of moles of the substance reacting.
- 😀 In the example of methane combustion, the enthalpy change was calculated as -801.5 kJ/mol, indicating that heat is released during the reaction.
- 😀 In neutralization reactions, like between NaOH and HCl, the enthalpy change can also be calculated by considering the heat absorbed by the solution and the number of moles involved.
- 😀 The specific heat capacity of water is essential in calorimeter calculations, and when dealing with simple calorimeters, any heat absorbed by the calorimeter itself is often neglected.
Q & A
What is the main purpose of a calorimeter in chemical reactions?
-The main purpose of a calorimeter is to measure the heat change (enthalpy change) during chemical reactions, specifically the heat released or absorbed by the system.
What are the two main types of calorimeters discussed in the video?
-The two main types of calorimeters discussed are the bomb calorimeter and the simple calorimeter. The bomb calorimeter is used for combustion reactions, while the simple calorimeter is typically used for reactions like mixing solutions.
How does a bomb calorimeter work?
-A bomb calorimeter works by burning a substance in a sealed chamber (the bomb). The heat released from the reaction is absorbed by the surrounding water and the calorimeter. The temperature change in the water is used to calculate the heat released.
What is the formula to calculate the heat change in water?
-The formula to calculate the heat change in water is Q = mcΔT, where m is the mass of water, c is the specific heat capacity of water, and ΔT is the temperature change.
What is the difference between a bomb calorimeter and a simple calorimeter?
-A bomb calorimeter is used for high-energy reactions, such as combustion, where the substance is burned in a sealed bomb. A simple calorimeter, typically a styrofoam cup, is used for reactions where heat is transferred to or from a solution, such as mixing two liquids.
What role does the stirrer play in a bomb calorimeter?
-The stirrer in a bomb calorimeter helps to evenly distribute the heat generated from the combustion throughout the surrounding water, ensuring a uniform temperature change.
How do you calculate the total heat change during a reaction in a bomb calorimeter?
-The total heat change is the sum of the heat absorbed by the water and the heat absorbed by the calorimeter. The formula is: Total Heat Change = Q_water + Q_calorimeter.
What does the formula Q = CΔT represent in the context of a calorimeter?
-The formula Q = CΔT represents the heat change in the calorimeter itself, where C is the heat capacity of the calorimeter and ΔT is the temperature change.
Why is it important to calculate enthalpy change (ΔH) in reactions?
-Calculating enthalpy change (ΔH) is important because it helps determine the energy released or absorbed during a chemical reaction. It is crucial for understanding the energy efficiency of processes and reactions.
In the example of the combustion of methane, how is the enthalpy change (ΔH) calculated?
-In the example, the total heat released is first calculated by adding the heat absorbed by the water and the calorimeter. Then, the enthalpy change (ΔH) is calculated by dividing the total heat change by the number of moles of methane combusted.
What does the term 'negative ΔH' indicate in a chemical reaction?
-A negative ΔH indicates that the reaction is exothermic, meaning it releases heat to the surroundings.
Outlines

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantMindmap

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantKeywords

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantHighlights

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantTranscripts

Cette section est réservée aux utilisateurs payants. Améliorez votre compte pour accéder à cette section.
Améliorer maintenantVoir Plus de Vidéos Connexes

PRAKTIKUM PENENTUAN PERUBAHAN ENTALPI REAKSI PENETRALAN

Termokimia part 4- HUKUM HESS - Kimia SMA kelas 11 semester 1

PENENTUAN ENTALPI REAKSI DENGAN KALORIMETER
Bomb Calorimeter vs Coffee Cup Calorimeter Problem - Constant Pressure vs Constant Volume Calorimet

PERCOBAAN KALORIMETRI (PRAKTIKUM KALORIMETER SEDERHANA)
Calorimetry
5.0 / 5 (0 votes)
