The Extraction of Iron (GCSE Chemistry)
Summary
TLDRThis educational video provides an in-depth explanation of the extraction of iron from its ore using the blast furnace process. It covers key reactions involved, including combustion, reduction, decomposition, and neutralization. The process uses raw materials like hematite, coke, limestone, and hot air to produce molten iron and slag. The video explains the role of each material and the chemical reactions taking place in the furnace, such as the reduction of iron oxide by carbon monoxide. It also touches on the importance of steel production and the role of carbon content in determining the properties of steel, such as hardness and brittleness.
Takeaways
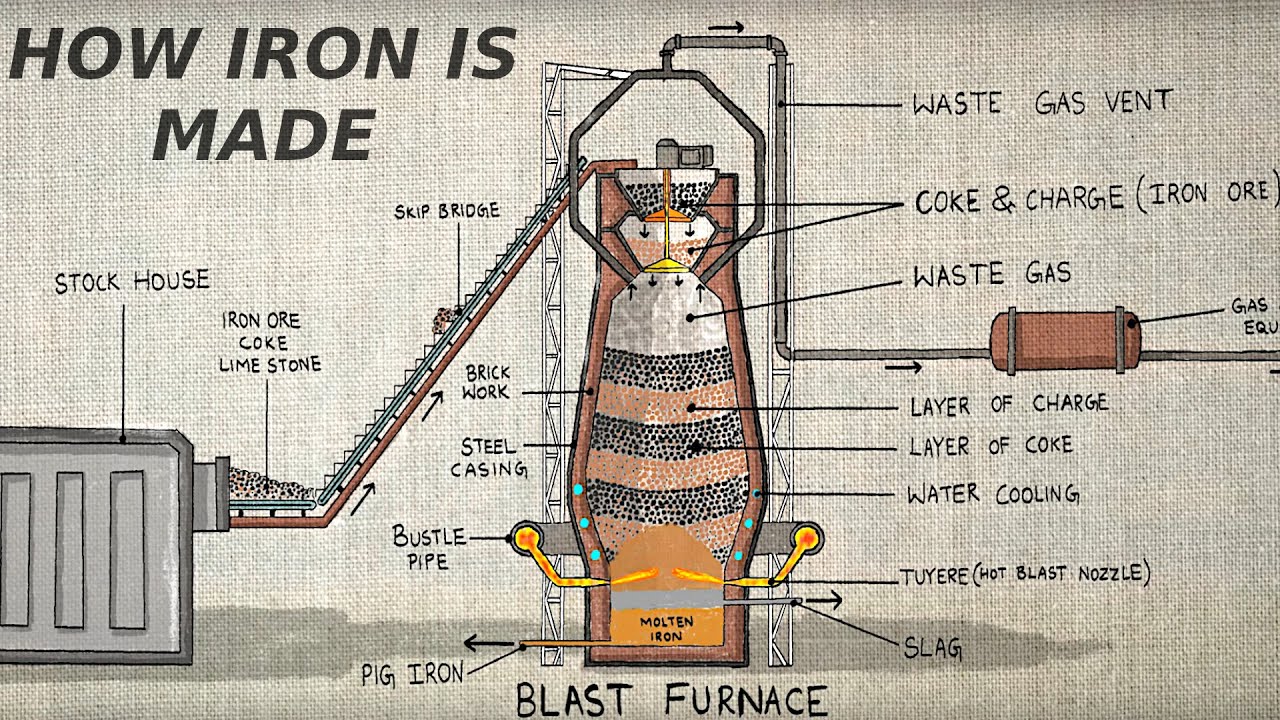
- 😀 Iron is extracted from iron ore using the blast furnace process, a critical industrial process for producing iron and steel.
- 😀 The main raw materials for the blast furnace are iron ore (hematite), coke, limestone, and hot air.
- 😀 Coke serves both as a fuel to generate high temperatures and as a reducing agent that helps in the reduction of iron ore to iron.
- 😀 The blast furnace operates at extremely high temperatures (around 1500°C), allowing the necessary chemical reactions to take place.
- 😀 Combustion reactions occur when coke burns in oxygen to form carbon dioxide and release heat energy.
- 😀 Carbon monoxide, formed from the reaction of carbon dioxide with more coke, acts as the reducing agent in the furnace, reducing iron oxide to iron.
- 😀 Limestone (calcium carbonate) is used to remove impurities from the iron ore, specifically by reacting with silica to form slag.
- 😀 Slag, a byproduct of the blast furnace process, is formed from the combination of calcium oxide and silica and can be used in road building and concrete.
- 😀 The process includes both reduction (iron oxide loses oxygen) and oxidation (carbon monoxide gains oxygen) reactions.
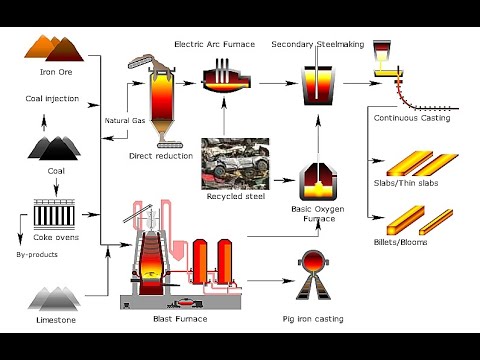
- 😀 Iron produced in the blast furnace is impure, containing 3-4% carbon, and is known as cast iron, which is brittle and unsuitable for many applications.
- 😀 Steel, an alloy of iron and other elements, is produced by removing excess carbon from the iron and adjusting its properties by adding elements like chromium and nickel.
Q & A
What is the role of coke in the blast furnace process?
-Coke acts as both a fuel and a reducing agent in the blast furnace. It burns in oxygen to produce heat, which is necessary for maintaining the high temperature of the furnace, and it also generates carbon monoxide, which reduces iron oxide (Fe2O3) to iron.
What are the key reactions occurring in the blast furnace?
-The key reactions are combustion (coke burns to form carbon dioxide), reduction (carbon monoxide reduces iron oxide to iron), and thermal decomposition (limestone breaks down to calcium oxide and carbon dioxide). Additionally, there is a neutralization reaction where calcium oxide reacts with silicon dioxide to form slag.
What is the purpose of limestone in the blast furnace?
-Limestone is used to remove impurities from iron ore. It breaks down into calcium oxide, which reacts with the silica (silicon dioxide) impurities in the iron ore to form slag, which can then be removed.
What does the term 'reducing agent' mean in the context of the blast furnace?
-A reducing agent is a substance that causes another substance to lose oxygen. In the blast furnace, carbon monoxide is the reducing agent because it causes iron oxide to lose oxygen and form iron.
Why is hot air used in the blast furnace?
-Hot air is blown into the blast furnace to provide the necessary oxygen for combustion. This helps the coke burn at very high temperatures (around 1500°C), which is essential for the chemical reactions to occur effectively.
What is the final product of the blast furnace process?
-The final products of the blast furnace are molten iron and slag. The iron is extracted as a liquid and can be further processed to make steel, while slag, formed from impurities, is removed and can be used in industries like road building and cement manufacturing.
What happens to the iron in the blast furnace after extraction?
-The iron extracted from the blast furnace is impure, containing around 3-4% carbon. This impure iron is then typically converted into steel, which is a mixture of iron and other elements, by removing the excess carbon and adding other metals to adjust its properties.
What are some uses of slag produced in the blast furnace?
-Slag, a byproduct of the blast furnace, has several uses including as a road-building material, aggregate in concrete, and even as a fertilizer due to its calcium and magnesium content.
How does the reactivity of metals relate to their extraction process?
-Metals at the bottom of the reactivity series are the easiest to extract, as they are found in their pure elemental form. Metals at the top of the series, such as potassium and sodium, are harder to extract because they are more stable in compounds and require electrolysis for extraction. Iron, which is in the middle of the reactivity series, can be extracted by reduction using a reducing agent like carbon monoxide.
How is the carbon content of steel adjusted during its production?
-The carbon content of steel is adjusted by blowing oxygen into the impure iron. This process burns off the excess carbon, reducing the brittleness of the steel. Additional elements, such as chromium or nickel, may also be added to alter the steel's properties, such as making it resistant to corrosion (e.g., stainless steel).
Outlines

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenMindmap

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenKeywords

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenHighlights

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführenTranscripts

Dieser Bereich ist nur für Premium-Benutzer verfügbar. Bitte führen Sie ein Upgrade durch, um auf diesen Abschnitt zuzugreifen.
Upgrade durchführen5.0 / 5 (0 votes)