Structure Elucidation from Spectroscopic Data in Organic Chemistry
Summary
TLDRIn this organic chemistry tutorial, the instructor demonstrates how to elucidate a molecular structure using IR, mass spectrometry, and NMR data. Starting with IR, they identify an alcohol functional group and rule out carbonyls. Mass spectrometry provides the molecular formula C₈H₁₀O and reveals four degrees of unsaturation, suggesting an aromatic ring. Carbon-13 NMR shows six unique carbons, indicating symmetry, while proton NMR identifies a methyl group, a methane proton, aromatic hydrogens, and an OH proton. By analyzing integrations and splitting patterns, the fragments are assembled to reveal a mono-substituted benzyl alcohol derivative, illustrating a systematic approach to structural determination.
Takeaways
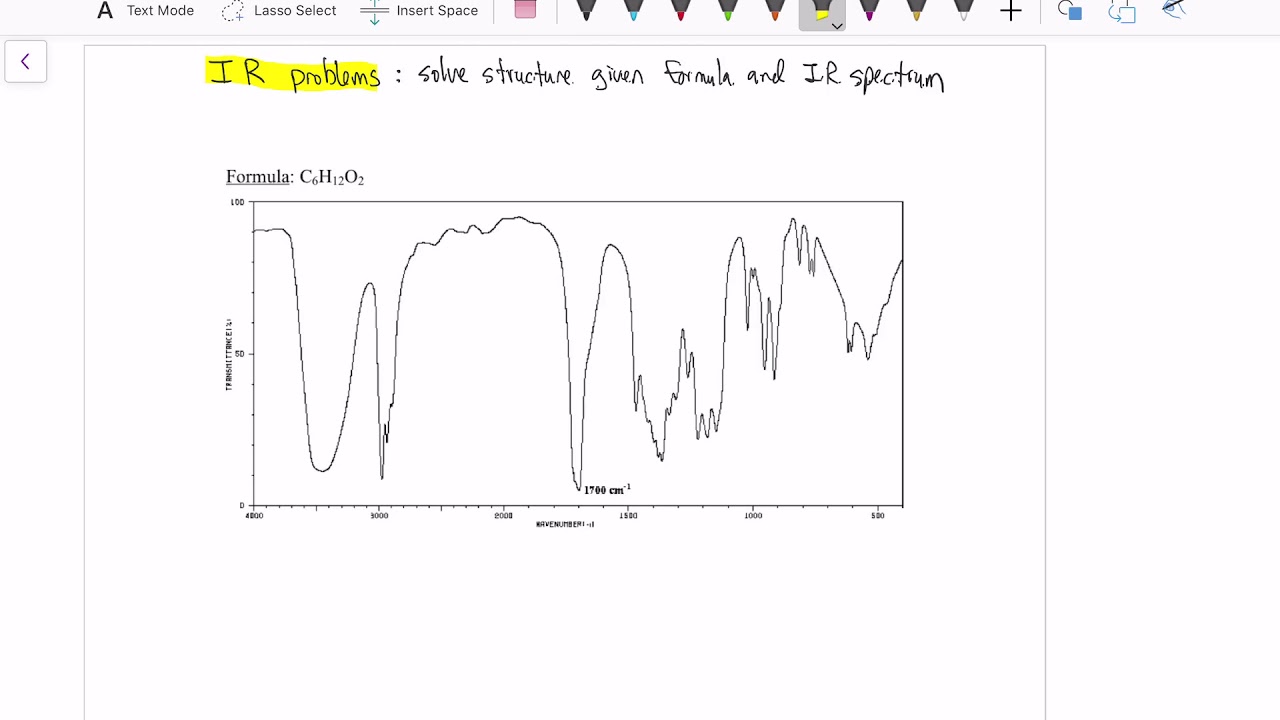
- 🔬 IR spectroscopy revealed a broad peak characteristic of an O-H stretch, indicating the presence of an alcohol.
- ❌ The absence of peaks between 1600–1800 cm⁻¹ in the IR spectrum confirms no carbonyl groups are present.
- 🧪 Mass spectrometry provided the molecular formula C8H10O, allowing for further structural analysis.
- 📏 Degrees of unsaturation were calculated as 4, suggesting a combination of rings and/or double bonds in the molecule.
- 🧩 Carbon-13 NMR showed six unique carbon signals, indicating some molecular symmetry.
- 🔹 The carbon chemical shifts identified four sp2 hybridized carbons, consistent with an aromatic benzene ring.
- 📊 Proton NMR revealed four unique sets of hydrogens, helping determine the relative positions and types of hydrogen atoms.
- 🧠 Integration of the proton NMR peaks indicated a methyl group, a methine group, and a mono-substituted benzene ring.
- -
- 🔗 Coupling patterns in the proton NMR (doublet and quartet) helped identify neighboring hydrogen interactions and bond connectivity.
- -
- 🖋️ By combining IR, mass spec, carbon-13 NMR, and proton NMR data, the final structure was elucidated as a benzene ring with a methanol and methyl substituent (C8H10OH).
- -
- ✅ This tutorial demonstrated a systematic approach to deducing molecular structure from multiple spectroscopic techniques.
Q & A
What functional group is indicated by the broad IR peak in the 3200–3600 cm⁻¹ region?
-The broad IR peak in this region indicates the presence of an O–H group, which is characteristic of alcohols.
Why is there no carbonyl group in the molecule based on the IR spectrum?
-There is no significant peak between 1600–1800 cm⁻¹ in the IR spectrum, which is the typical region for C=O stretches, indicating the absence of carbonyl groups.
How is the degree of unsaturation calculated for C8H10O?
-The degree of unsaturation is calculated using the formula DU = (H_saturated – H_actual)/2. For C8H10O, a saturated alkane would have 18 H; the molecule has 10 H, so DU = (18-10)/2 = 4.
What does a degree of unsaturation of 4 tell us about the molecule?
-A degree of unsaturation of 4 suggests the molecule contains a combination of rings and/or π bonds, such as a benzene ring (3 π bonds) and one additional ring or double bond.
How many unique carbons are observed in the 13C NMR spectrum, and what does this imply?
-Six unique carbon signals are observed, implying some symmetry in the molecule since the molecular formula has 8 carbons.
What evidence in the 13C NMR spectrum indicates the presence of an aromatic ring?
-Four carbons appear in the sp² region typical of aromatic carbons, indicating a benzene ring is present.
How are the proton NMR integrations interpreted for C8H10O?
-The integrations correspond to 5H (aromatic), 3H (methyl), 1H (methine), and 1H (hydroxyl). This helps assign the types of hydrogen atoms in the molecule.
What does the splitting pattern of the methyl group in the 1H NMR indicate?
-The methyl group appears as a doublet, indicating it is coupled to a single neighboring proton (CH) according to the n+1 rule.
How is the hydroxyl proton identified in the 1H NMR spectrum?
-The hydroxyl proton appears as a singlet that exchanges with D2O, confirming it is bonded to an oxygen atom.
What is the final proposed structure of the molecule C8H10O based on the combined spectroscopic data?
-The final structure is a mono-substituted benzene ring with a CH–CH3 fragment bearing a hydroxyl group: C6H5–CH(OH)–CH3 (1-phenylethanol).
Why is the benzene ring considered mono-substituted in this molecule?
-The 1H NMR shows a single set of aromatic protons integrating to 5H, which is characteristic of a mono-substituted benzene ring.
How do the IR, 1H NMR, 13C NMR, and mass spectrometry data complement each other in structure elucidation?
-IR identifies functional groups (alcohol), NMR provides carbon and hydrogen environments and coupling patterns, and mass spectrometry confirms the molecular formula and degrees of unsaturation. Together, they allow the accurate assembly of the molecule's structure.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة
Determine structures from IR spectra

Espectrometria de Massas (Vídeo 1: Instrumentação)
IR Spectroscopy and Mass Spectrometry: Crash Course Organic Chemistry #5

CARA MUDAH BACA SPEKTRA IR, MS, HNMR dan CNMR SENYAWA ORGANIK

Cara mudah baca spektra IR | Bahas soal spektra IR | Kupas tuntas 5 soal spektra IR

NMR spectroscopy
5.0 / 5 (0 votes)
