Termodinamika Kelas XI IPA
Summary
TLDRThis educational video provides an in-depth introduction to thermodynamics, covering fundamental concepts such as energy transfer, heat, and work between systems and their surroundings. It explores key thermodynamic laws, including the Zeroth, First, and Second Laws, and explains different types of systems (open, closed, and isolated). Practical applications, such as the Carnot cycle, refrigeration, and processes like isothermal, isobaric, isochoric, and adiabatic, are discussed. The video highlights real-life examples of these processes, making complex thermodynamic principles accessible and engaging for learners.
Takeaways
- 😀 Thermodynamics is the branch of physics that studies energy transfer as heat and work between systems and their surroundings.
- 😀 A system refers to the specific part of the universe being observed, while the surroundings include everything outside of the system that interacts with it.
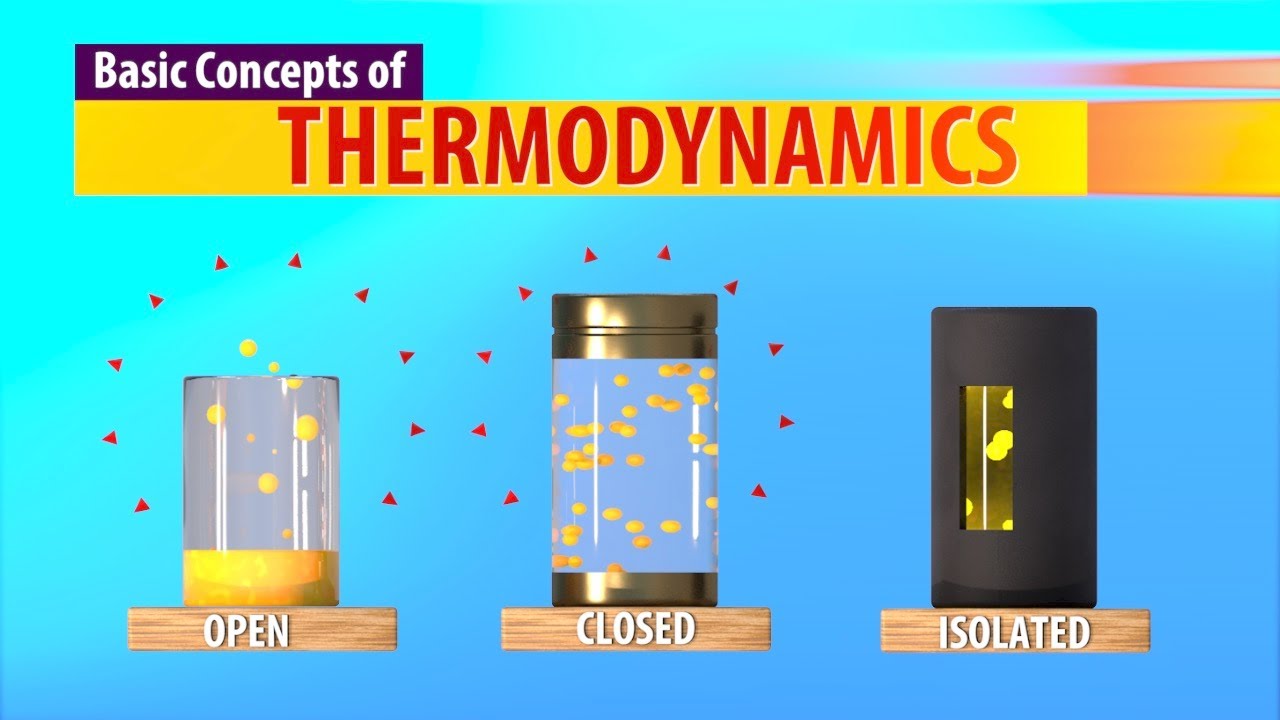
- 😀 There are three main types of systems: open systems (energy and matter exchange), closed systems (energy exchange only), and isolated systems (no exchange of energy or matter).
- 😀 The Zeroth Law of Thermodynamics states that if two objects are in thermal equilibrium with a third object, they are in thermal equilibrium with each other.
- 😀 The First Law of Thermodynamics is the law of energy conservation: energy cannot be created or destroyed, only transferred as heat or work.
- 😀 The Second Law of Thermodynamics states that heat flows naturally from a hot object to a cold one, and no machine can convert heat into work with 100% efficiency.
- 😀 Thermodynamic processes include isothermal (constant temperature), isobaric (constant pressure), isochoric (constant volume), and adiabatic (no heat exchange).
- 😀 Real-life applications of thermodynamic processes include air conditioning (isothermal), steam engines (isobaric), fans in a closed system (isochoric), and thermos bottles (adiabatic).
- 😀 The Carnot Cycle, which involves isothermal, isobaric, and isochoric processes, represents the most efficient thermodynamic cycle and can be used to calculate engine efficiency.
- 😀 Entropy is a measure of the amount of heat energy in a system that cannot be converted into work, and it plays a crucial role in understanding energy transfer in thermodynamics.
Q & A
What is thermodynamics and where does the term originate from?
-Thermodynamics is a branch of physics that studies the processes of energy transfer as heat and work between systems and their surroundings. The term originates from Greek: 'thermos' meaning heat and 'dynamis' meaning power or change.
What are the two main components in thermodynamics?
-In thermodynamics, there are two main components: the system, which is the part of the universe being studied, and the surroundings, which is everything outside the system that can interact with it.
Can you explain the difference between an open, closed, and isolated system in thermodynamics?
-In an open system, both energy and matter can be exchanged with the surroundings. In a closed system, only energy can be exchanged, not matter. In an isolated system, neither energy nor matter can be exchanged with the surroundings.
What is the first law of thermodynamics?
-The first law of thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed. It can only change form, and the total energy in a system is conserved.
How is the first law of thermodynamics mathematically expressed?
-The first law of thermodynamics is expressed as Q = ΔU + W, where Q is the heat added to the system, ΔU is the change in internal energy, and W is the work done by the system.
What is an example of an application of the zeroth law of thermodynamics?
-An example of the zeroth law of thermodynamics is when water is heated in a pot. When the temperature of the pot and water equalize, they reach thermal equilibrium, meaning no heat flows between them. This is an application of the zeroth law.
What does the second law of thermodynamics state about heat flow?
-The second law of thermodynamics states that heat naturally flows from a hotter object to a cooler one, and it will not flow spontaneously in the opposite direction without external work being applied.
What is a Carnot cycle and what does it demonstrate in thermodynamics?
-The Carnot cycle is a theoretical thermodynamic cycle that demonstrates the maximum possible efficiency of a heat engine. It involves isothermal, isobaric, and isochoric processes and shows how heat can be converted into work with optimal efficiency.
What is the significance of entropy in thermodynamics?
-Entropy is a measure of the amount of disorder or randomness in a system. In thermodynamics, it represents the energy in a system that is unavailable to do work. It is used to quantify the irreversibility of a process.
How is the efficiency of a Carnot engine calculated?
-The efficiency of a Carnot engine is calculated using the formula: Efficiency = (T_high - T_low) / T_high, where T_high is the temperature of the hot reservoir and T_low is the temperature of the cold reservoir. The efficiency is expressed as a percentage.
Outlines

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنMindmap

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنKeywords

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنHighlights

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنTranscripts

هذا القسم متوفر فقط للمشتركين. يرجى الترقية للوصول إلى هذه الميزة.
قم بالترقية الآنتصفح المزيد من مقاطع الفيديو ذات الصلة

11 02 Fisika Dasar 1- Termodinamika

Termodinamika - Fisika Kelas 11 (Kurikulum 2013 Revisi) - Quipper Video

Hukum Termodinamika, Bagian 1: Energi Dalam dan Hukum Pertama

VIDEO PEMBELAJARAN - FISIKA - HUKUM I TERMODINAMIKA

THERMODYNAMICS Basic Units and Pressure Concepts in 11 Minutes!
Basic Concepts of Thermodynamics (Animation)
5.0 / 5 (0 votes)
