PERSAMAAN TERMOKIMIA | CARA MENULIS PERSAMAAN TERMOKIMIA | PAK BOSS Channel | Cerdas Kimia |
Summary
TLDRIn this chemistry lesson, Pak Bos explains the concept of thermochemistry, focusing on changes in heat or enthalpy during chemical and physical processes. The video covers the differences between exothermic and endothermic reactions, illustrated by practical examples like detergent and urea in water. The lesson also details how to write thermochemical equations, emphasizing the importance of enthalpy changes (delta H) in reactions. Through various examples, such as the combustion of hydrocarbons and the formation or decomposition of substances, the video educates viewers on how to interpret and balance thermochemical equations. Pak Bos concludes by previewing upcoming topics in thermochemistry.
Takeaways
- 😀 Thermochemistry is the study of heat changes during chemical and physical processes.
- 😀 A thermochemical equation includes the enthalpy change (ΔH) alongside the chemical reaction.
- 😀 The key difference between a regular chemical equation and a thermochemical equation is the inclusion of ΔH, which can be either positive or negative.
- 😀 A negative ΔH indicates an exothermic reaction, where heat is released into the surroundings.
- 😀 A positive ΔH indicates an endothermic reaction, where heat is absorbed from the surroundings.
- 😀 Exothermic reactions, like the dissolution of detergent in water, release heat, which increases the temperature of the surrounding medium.
- 😀 Endothermic reactions, such as dissolving urea in water, absorb heat, which lowers the temperature of the surrounding medium.
- 😀 Thermochemistry also involves the concept of systems and surroundings: the system is the substance undergoing the reaction, while the surroundings are the rest of the environment affected by the reaction.
- 😀 The formula for ΔH is: ΔH = enthalpy of products - enthalpy of reactants.
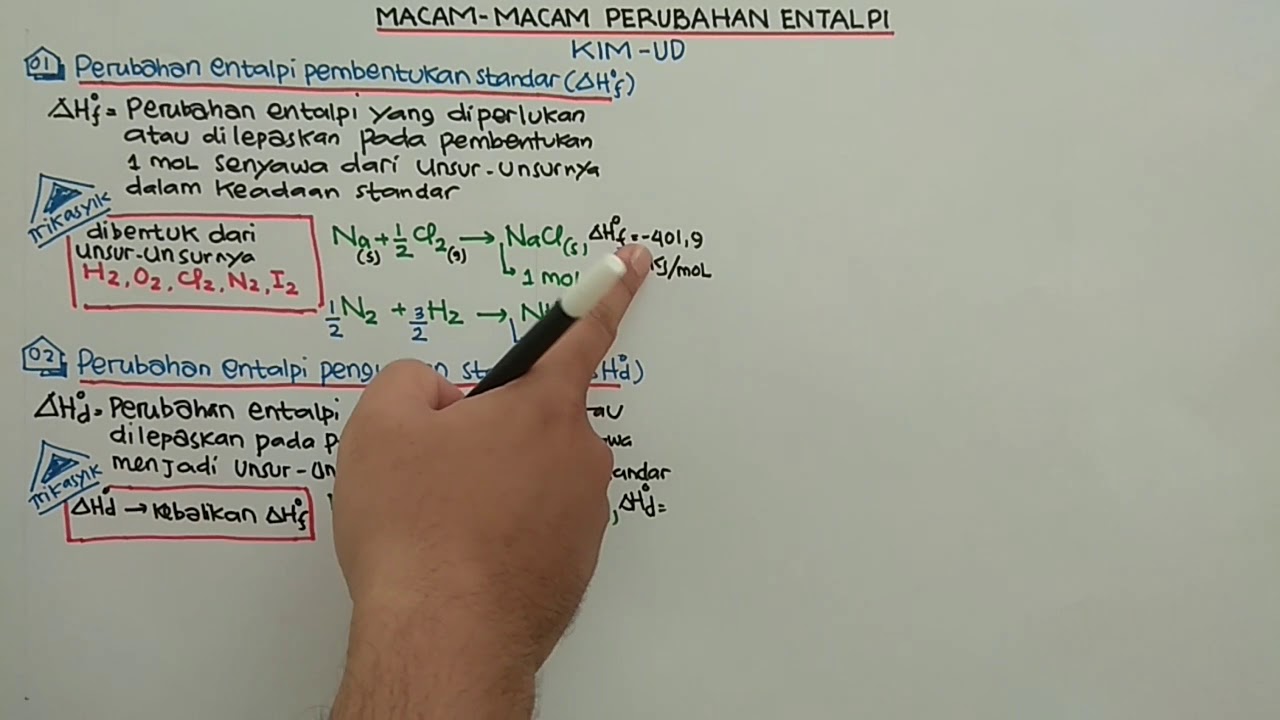
- 😀 Standard enthalpy changes include formation, decomposition, and combustion, each with specific methods for writing the corresponding thermochemical equations.
- 😀 The script explains how to write thermochemical equations for reactions like the formation of CO2, the decomposition of NaHCO3, and the combustion of CH4, highlighting the importance of balancing the equations and assigning the correct enthalpy changes.
Q & A
What is thermochemistry?
-Thermochemistry is the study of the heat changes that occur during chemical and physical processes. It involves understanding the heat energy changes, measured as enthalpy, during reactions.
How does thermochemistry differ from basic chemical equations?
-In thermochemistry, chemical equations include changes in enthalpy (denoted as delta H), while basic chemical equations only describe the substances involved in the reaction without considering energy changes.
What is the significance of the sign of delta H in thermochemical reactions?
-The sign of delta H indicates whether a reaction releases or absorbs heat. A negative delta H means the reaction releases heat (exothermic), while a positive delta H indicates heat absorption (endothermic).
What is an exothermic reaction, and can you provide an example?
-An exothermic reaction is one that releases heat into the surroundings. An example is the dissolution of detergent in water, which increases the temperature of the water.
What is an endothermic reaction, and can you provide an example?
-An endothermic reaction absorbs heat from the surroundings. An example is the dissolution of urea in water, which causes the water temperature to decrease.
How can we measure the temperature change in a thermochemical reaction?
-Temperature changes in thermochemical reactions can be measured using a thermometer to detect the rise or fall in temperature of the surrounding substance, such as water.
What is the relationship between system and surroundings in thermochemistry?
-In thermochemistry, the system is the substance being studied (such as the reactant), and the surroundings are everything else (such as the water or container). The system can absorb or release heat, affecting the temperature of the surroundings.
How is delta H calculated in thermochemistry?
-Delta H is calculated as the difference between the enthalpy of the products and the enthalpy of the reactants in a reaction. If the products have higher enthalpy than the reactants, delta H is positive (endothermic). If the reactants have higher enthalpy, delta H is negative (exothermic).
What is enthalpy of formation, and how is it written in thermochemical equations?
-Enthalpy of formation (delta Hf) is the heat change when one mole of a compound is formed from its elements in their standard states. In thermochemical equations, delta Hf is included, and it is typically negative for exothermic formation reactions.
Can you explain how the enthalpy of decomposition is represented in thermochemical equations?
-The enthalpy of decomposition (delta Hd) refers to the heat change when a compound breaks down into its elements. It is represented with a positive delta Hd in thermochemical equations because it requires energy (endothermic process).
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
5.0 / 5 (0 votes)