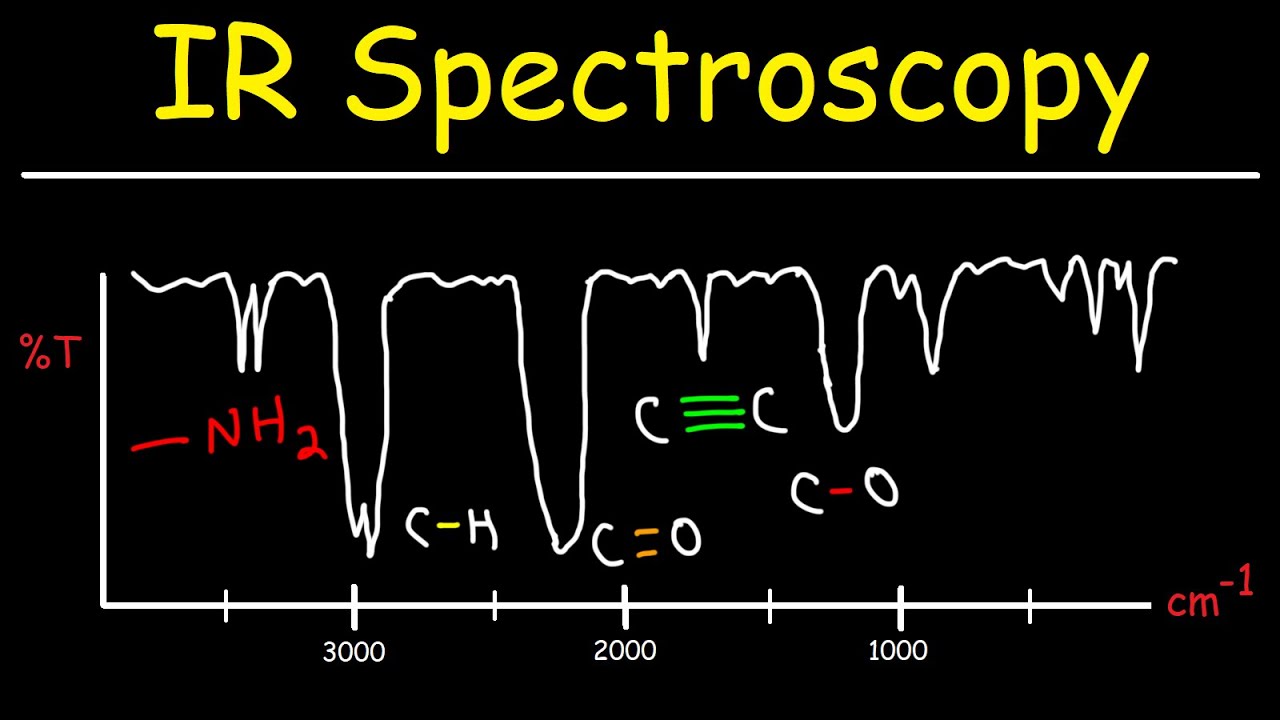
How to Interpret an IR Spectrum and Identify the RIGHT Functional Group
Summary
TLDRThis video provides a step-by-step guide to identifying functional groups in infrared (IR) spectroscopy. It covers key functional groups such as ketones, amines, carboxylic acids, and alcohols, explaining how to interpret specific peaks in the IR spectrum. Key identifiers include the position of carbonyl stretches for ketones, the number of peaks for amines, and broad versus narrow OH stretches for carboxylic acids and alcohols. The video emphasizes the importance of understanding peak shapes and regions to correctly identify functional groups, with practical tips and examples to clarify the process.
Takeaways
- 😀 Ketones are identified by a sharp peak around 1700 cm⁻¹ for the carbonyl stretch and typically only have one peak.
- 😀 Amines have a characteristic N-H stretching range between 3300-3500 cm⁻¹, with primary amines showing two peaks and secondary amines showing one peak.
- 😀 Alcohols show a broad O-H stretch in the 3200-3550 cm⁻¹ range, which helps distinguish them from other functional groups.
- 😀 Carboxylic acids exhibit both a broad O-H stretch (2500-3500 cm⁻¹) and a sharp carbonyl stretch near 1700 cm⁻¹.
- 😀 Alkyl groups (sp³ hybridized) show peaks around 2800-3000 cm⁻¹ due to C-H stretches, which can sometimes be confused with functional group peaks.
- 😀 The key to identifying functional groups lies in the region above 1400 cm⁻¹, the 'functional group region'.
- 😀 Secondary amines only show one peak in the 3300-3500 cm⁻¹ range, while primary amines show two.
- 😀 When identifying alcohols and carboxylic acids, focus on the broadness of the O-H stretch; carboxylic acids have a more 'fat' broad stretch.
- 😀 Ketones are not easily confused with other groups because they have only one peak in the carbonyl region and no additional peaks like aldehydes or acids.
- 😀 The difference between an alcohol and a carboxylic acid lies in the additional sharp carbonyl peak for carboxylic acids, distinguishing them from alcohols.
Q & A
What is the functional group region in an IR spectrum?
-The functional group region in an IR spectrum refers to the area greater than 1400 cm⁻¹. This is where significant peaks for various functional groups like carbonyl (C=O), hydroxyl (O-H), and amines are observed.
How can you identify a ketone from an IR spectrum?
-A ketone can be identified by a distinct carbonyl stretch around 1700 cm⁻¹. It typically shows a single peak in this region, unlike other carbonyl-containing functional groups like carboxylic acids, which have additional peaks.
What feature distinguishes primary and secondary amines in an IR spectrum?
-The main distinguishing feature between primary and secondary amines is the number of N-H peaks. A primary amine has two peaks due to two N-H bonds, while a secondary amine shows only one peak due to a single N-H bond.
How can you tell the difference between alcohols and carboxylic acids in an IR spectrum?
-Alcohols and carboxylic acids both show broad peaks around 3200-3550 cm⁻¹, but carboxylic acids also have a sharp carbonyl stretch around 1700 cm⁻¹ and a broader O-H stretch, which makes them distinguishable from alcohols.
Why is the fingerprint region below 1400 cm⁻¹ usually not useful in functional group identification?
-The fingerprint region contains many complex peaks that are often unique to the molecule and specific to the structure. It is difficult to assign these peaks to specific functional groups, which is why the focus is usually on the functional group region above 1400 cm⁻¹.
What is the significance of the 3000 cm⁻¹ region in an IR spectrum?
-The 3000 cm⁻¹ region is typically associated with the C-H stretch of sp³ hybridized carbons. This range can be found in many functional groups, such as alkyl chains, but it is not specific to any particular functional group, so it requires further analysis.
What makes a peak broad in the IR spectrum, such as the O-H stretch in alcohols and carboxylic acids?
-A broad peak is often the result of hydrogen bonding, which causes a widening of the absorption band. In alcohols and carboxylic acids, the O-H stretch appears as broad due to the presence of hydrogen bonding interactions.
How can the presence of an amine be confirmed in an IR spectrum?
-The presence of an amine is confirmed by a peak in the 3000-3500 cm⁻¹ range, where the N-H stretch occurs. Primary amines show two peaks, while secondary amines show only one peak.
What is the main characteristic of a carboxylic acid in an IR spectrum?
-A carboxylic acid is characterized by a broad O-H stretch around 2500-3300 cm⁻¹ and a sharp carbonyl stretch around 1700 cm⁻¹. The broadness of the O-H stretch, combined with the carbonyl stretch, is a key identifier.
How can an alcohol be distinguished from a carboxylic acid in an IR spectrum?
-An alcohol and a carboxylic acid both have a broad O-H stretch, but a carboxylic acid also shows a sharp C=O stretch around 1700 cm⁻¹, which is not present in alcohols. The broadness of the O-H stretch in a carboxylic acid is also typically more pronounced.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级浏览更多相关视频
5.0 / 5 (0 votes)