2.2/S1.3.2 The Line Spectrum of Hydrogen [SL IB Chemistry]
Summary
TLDRThe video explores the line spectra of hydrogen using a spectroscope, detailing how electrons transition between discrete energy levels to emit photons of light. As energy is added to hydrogen, electrons can jump to higher levels and subsequently release energy as they fall back, creating a unique spectrum for hydrogen. The speaker emphasizes the convergence of spectral lines at high energy and the significance of these spectra in identifying elements, including the historical discovery of helium in the Sun. The application of these principles in space exploration, such as the Curiosity rover's analysis of Martian rocks, is also highlighted.
Takeaways
- 😀 A spectroscope allows us to observe the line spectra of elements, such as hydrogen.
- 🔬 The line spectrum shows selected frequencies of light emitted by excited electrons in an atom.
- ⚛️ Hydrogen's line spectrum consists of distinct lines corresponding to specific energy transitions of electrons.
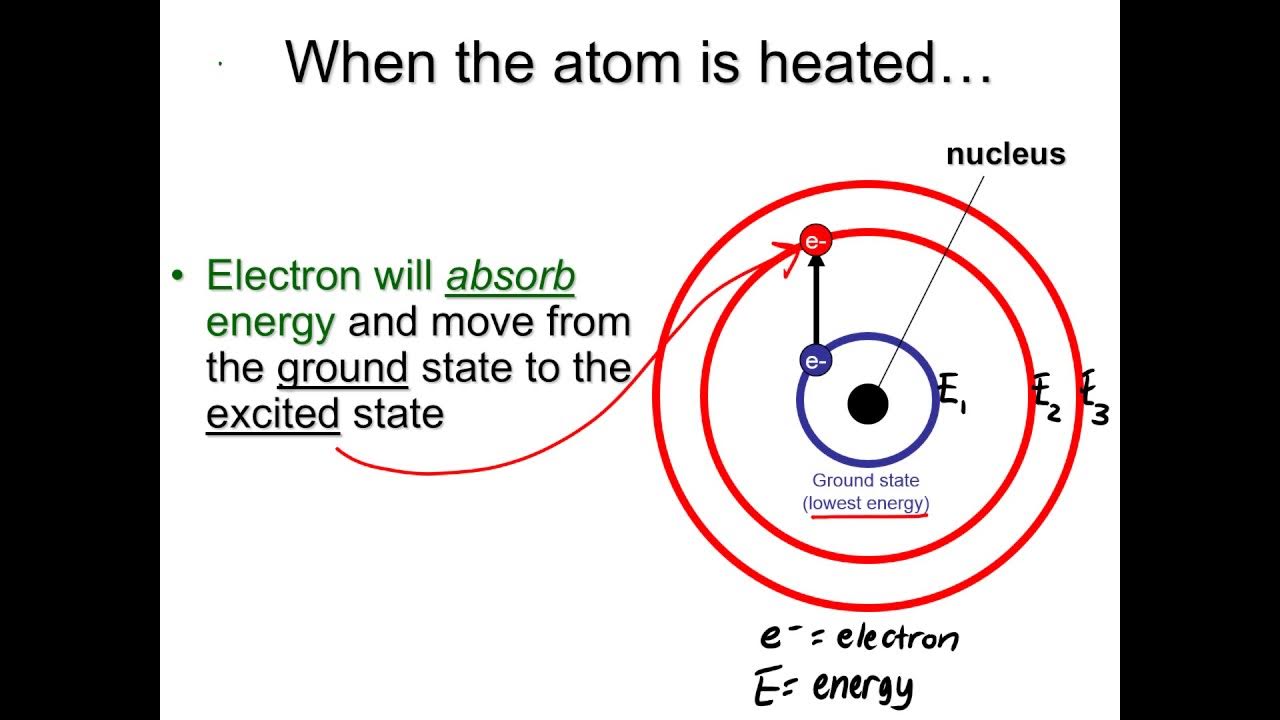
- 🌌 Electrons can only exist in certain energy levels, which are quantized and represented as concentric rings around the nucleus.
- 💡 When energy is added to an electron, it can jump to a higher energy level (excitation) and eventually release a photon when it returns to a lower level.
- 🔄 Larger energy jumps result in the emission of higher energy photons, such as ultraviolet light, while smaller jumps emit lower energy photons like visible light and infrared.
- 📈 The energy levels converge as they move away from the nucleus, which is why the lines on the line spectrum also converge at high energy.
- 🌠 The unique line spectra of elements can help identify the presence of specific elements in various environments, such as in stars.
- 🌞 Helium was first discovered in the Sun using spectroscopy before being identified on Earth.
- 🚀 Modern instruments, like the spectrometer on NASA's Curiosity rover, analyze the line spectra of rocks on Mars to identify their atomic composition.
Q & A
What is a spectroscope used for?
-A spectroscope is used to analyze the line spectra of elements, such as hydrogen, by showing specific frequencies of light emitted when electrons transition between energy levels.
How does the electron's movement between energy levels contribute to the line spectra?
-When an electron absorbs energy, it can jump from an inner shell to an outer shell. When it falls back to a lower energy level, it releases a photon of light, which corresponds to a specific frequency, contributing to the line spectra.
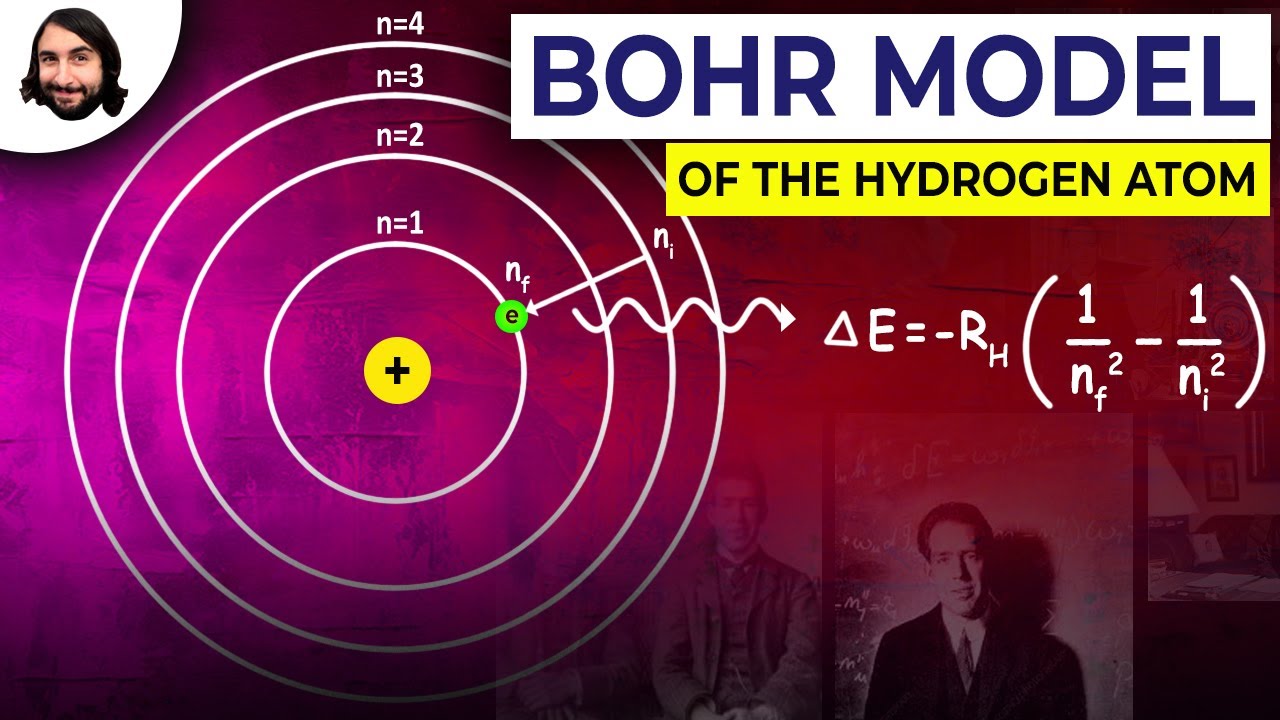
What are the main energy levels for the hydrogen atom mentioned in the script?
-The energy levels for the hydrogen atom are labeled as n = 1, n = 2, n = 3, and so on, with n = 1 being the closest to the nucleus.
What happens when an electron is excited to a higher energy level?
-When an electron is excited to a higher energy level, it may jump up one or more levels. This excited state is temporary, and the electron will eventually return to a lower energy level, emitting a photon in the process.
What types of light correspond to transitions between different energy levels?
-Transitions from n = 1 result in ultraviolet light, transitions from n = 2 result in visible light, and transitions from n = 3 result in infrared light.
How do the energy levels behave as they get further from the nucleus?
-The energy levels are concentric and converge, meaning that as you move further from the nucleus, the energy levels become closer together.
What does it mean for a jump to result in high-energy photons?
-A larger jump from one energy level to another corresponds to a higher energy photon being emitted. The energy of the photon released is proportional to the distance of the jump.
What significant discovery was made using line spectra in the context of celestial observations?
-Helium was discovered in the Sun using line spectra before it was found on Earth, as researchers observed colors and spectral lines not associated with any known elements.
What role does the laser play in the Curiosity rover's analysis of Martian rocks?
-The laser shoots at rocks, exciting the atoms within them. The spectrometer then analyzes the emitted line spectra to identify the elements present in the rocks.
Why is the ability to see line spectra important for identifying elements?
-Each element has a unique line spectrum, allowing scientists to identify which elements are present in a sample by analyzing the specific wavelengths of light emitted.
Outlines

此内容仅限付费用户访问。 请升级后访问。
立即升级Mindmap

此内容仅限付费用户访问。 请升级后访问。
立即升级Keywords

此内容仅限付费用户访问。 请升级后访问。
立即升级Highlights

此内容仅限付费用户访问。 请升级后访问。
立即升级Transcripts

此内容仅限付费用户访问。 请升级后访问。
立即升级5.0 / 5 (0 votes)