FISIKA ATOM 02_SPEKTRUM ATOM HIDROGEN
Summary
TLDRIn this video, the speaker delves into the physics of hydrogen atom spectra, specifically focusing on energy transitions between electron shells. The explanation covers Niels Bohr's atomic model, where electrons absorb or emit energy when moving between energy levels, leading to the emission of electromagnetic waves. The video explores the formulas for calculating energy changes and provides examples. The speaker also introduces the five main series of hydrogen spectra, including the Lyman, Balmer, and other series, discussing how to calculate wavelengths of emitted radiation. Overall, the video offers a clear and detailed explanation of the hydrogen atom’s behavior and its spectral lines.
Takeaways
- 😀 The video discusses the spectrum of the hydrogen atom and its various series, building on Bohr's atomic model.
- 😀 The concept of electron energy levels in hydrogen atoms is explained, with electrons transitioning between energy shells.
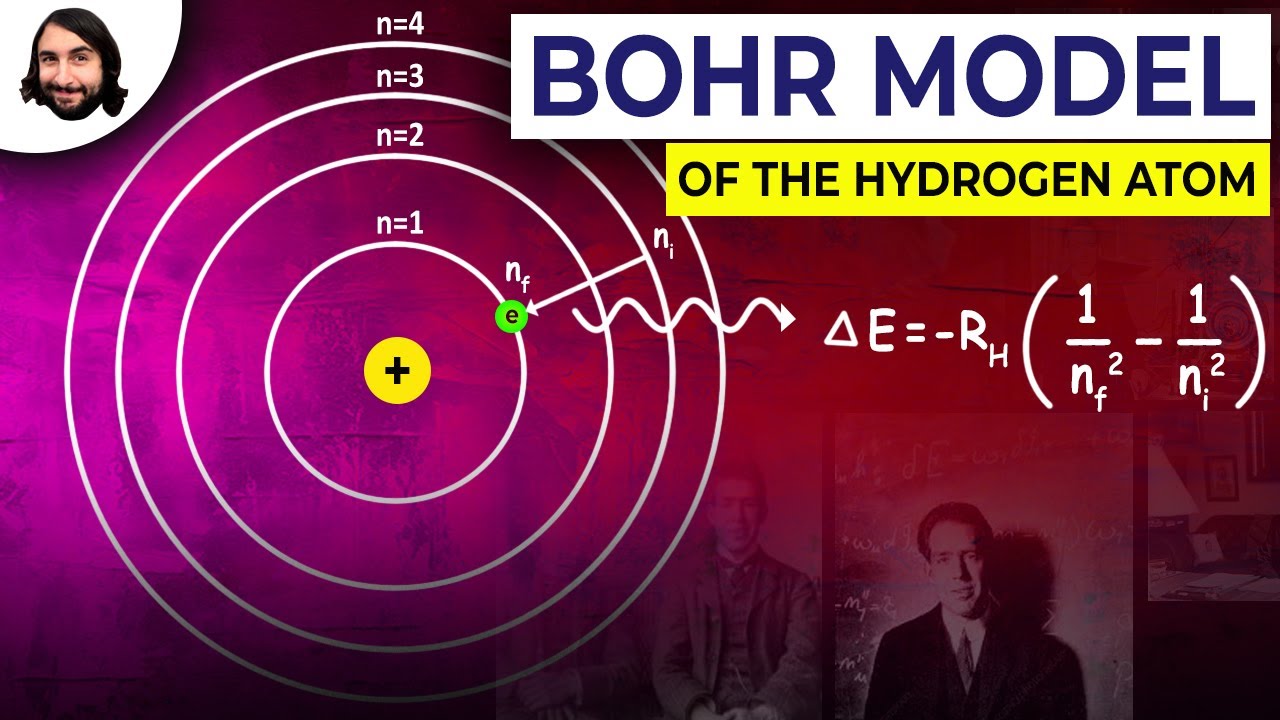
- 😀 When an electron moves to a higher orbit, it absorbs energy; when it moves to a lower orbit, it emits energy in the form of electromagnetic waves.
- 😀 The energy change (ΔE) during electron transitions is given by the equation ΔE = h*f, where h is Planck's constant and f is frequency.
- 😀 The energy of electrons in specific shells can be calculated using the formula: E = -13.6/n^2 eV, where n is the shell number.
- 😀 A positive value of ΔE indicates emission of energy, while a negative value indicates energy absorption by the atom.
- 😀 The video explores different spectral series in hydrogen atom transitions, such as the Lyman, Balmer, Paschen, Brackett, and Pfund series.
- 😀 The Lyman series corresponds to transitions where the electron moves to the first shell, emitting ultraviolet radiation.
- 😀 The Balmer series involves transitions to the second shell, producing visible light emissions.
- 😀 The formula for calculating the wavelength of emitted radiation from the hydrogen atom is given by λ = R * (1/n₁² - 1/n₂²), where n₁ and n₂ are the initial and final shell numbers, respectively.
- 😀 The video demonstrates how to calculate the wavelength for transitions in the Lyman series and explains the behavior of different transitions in terms of wavelength.
Q & A
What is the main topic of the video?
-The main topic of the video is the spectrum of the hydrogen atom and the energy transitions that occur within it.
What does Bohr's model of the hydrogen atom explain regarding electron movement?
-Bohr's model explains that electrons in an atom occupy specific orbits or shells and do not emit energy while in these stable orbits. However, they can absorb or emit energy when transitioning between different energy levels or shells.
How does energy absorption or emission occur when an electron transitions between orbits?
-When an electron moves from an outer orbit to a closer one, it emits energy in the form of electromagnetic radiation. Conversely, when it moves from an inner orbit to a more distant one, it absorbs energy.
What is the formula for calculating the energy change when an electron transitions between orbits?
-The energy change (ΔE) can be calculated using the formula ΔE = h * f, where h is Planck’s constant and f is the frequency of the emitted or absorbed radiation.
What is the significance of the value -13.6 eV in the energy equation?
-The value -13.6 eV represents the energy of an electron in the hydrogen atom's first orbit (n=1). It is used to calculate the energy of an electron in any other orbit using the formula E = -13.6 eV / n^2.
What is the meaning of a positive energy value when calculating the energy change?
-A positive energy value indicates that the atom is emitting energy, typically as electromagnetic radiation, when an electron transitions to a lower orbit.
What is the meaning of a negative energy value in the energy change equation?
-A negative energy value indicates that the atom is absorbing energy when the electron transitions to a higher orbit.
What are the different spectral series of the hydrogen atom discussed in the video?
-The video discusses five spectral series of the hydrogen atom: Lyman, Balmer, Paschen, Brackett, and Pfund. Each series corresponds to electron transitions ending at a specific orbit.
What is the Lyman series, and where do the electron transitions end?
-The Lyman series corresponds to transitions where the electron ends at the first orbit (n=1) and the transitions start from higher orbits (n=2, 3, 4, etc.). It was observed in 1906.
How is the wavelength of emitted radiation from these transitions calculated?
-The wavelength of the emitted radiation can be calculated using the formula λ = R * (1/n₁² - 1/n₂²), where λ is the wavelength, R is the Rydberg constant, n₁ is the target orbit, and n₂ is the initial orbit.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video
Bohr Model of the Hydrogen Atom

Fisika Atom • Part 2: Teori Atom Bohr

3.3.3 - Radiação eletromagnética: Teoria Quântica - Emissão de luz por gases excitados (Bohr)

Quantum Mechanics: Three Foundational Papers – Planck (1900), Einstein (1905), Bohr (1913)

51. Hydrogen Spectrum | Chapter 12 Atom | Physics Baba 2.0

Modelos de Rutherford e Bohr [Módulo 02 - Aula 02]
5.0 / 5 (0 votes)