Everyone should see this experiment for themselves
Summary
TLDRThe video explores Brownian motion, the random jiggling of particles suspended in gas or liquid, through various experiments capturing its effects visually. It explains how Einstein connected observations of Brownian motion to theories about atoms and molecules, using the measured motion of visible particles to calculate characteristics of invisible atoms. This allowed determination of Avogadro's number, enabling calculation of an atom's mass. The video ties this epiphany emerging from a complex natural phenomenon to the Jane Street program which similarly finds profound truths about finance, and encourages viewers to apply to this educational opportunity.
Takeaways
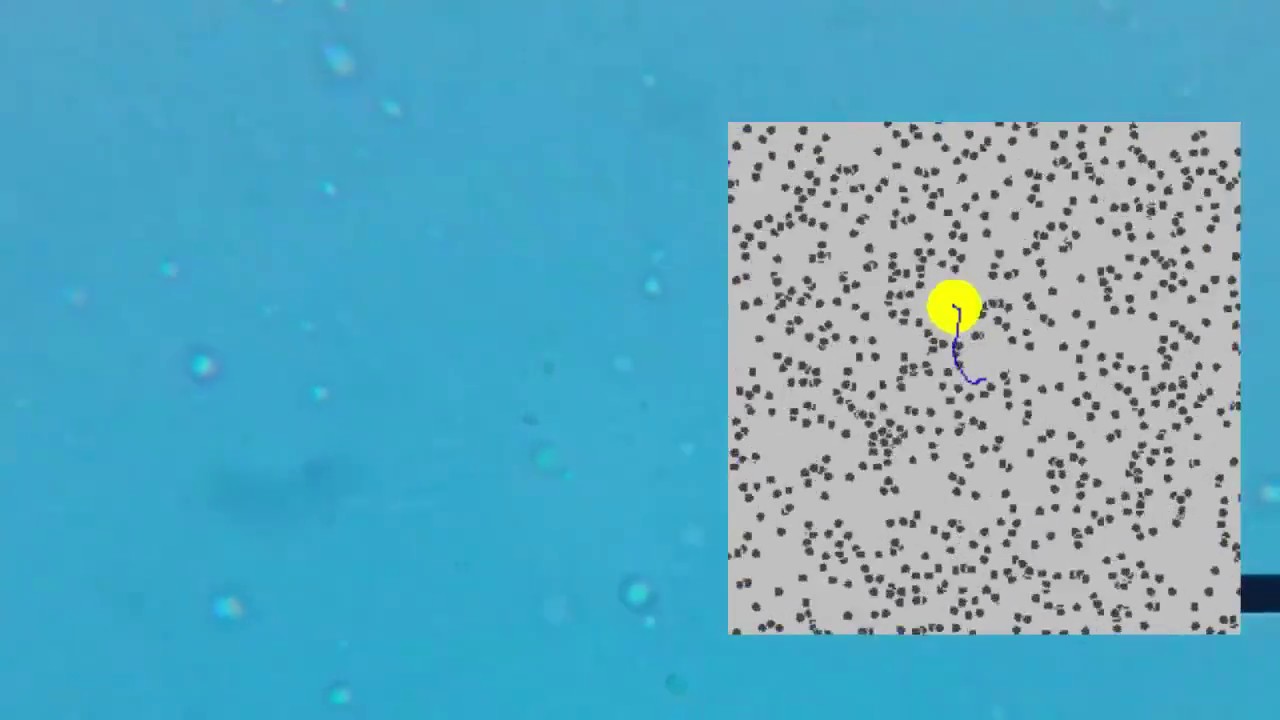
- 😀 Brownian motion is the random, jittery motion of microscopic particles suspended in a fluid, caused by collisions with atoms and molecules
- 🔬 Robert Brown first observed this motion in 1827 while looking at pollen grains under a microscope
- 💡 Albert Einstein realized he could use equations describing molecular motion to calculate properties of the visible particles exhibiting Brownian motion
- 📏 By observing Brownian motion, Einstein showed you could measure Avogadro's number and determine the mass of individual atoms
- 🔬 The script demonstrates Brownian motion using smoke, emulsions, milk and macroscopic models with ball bearings
- 🎥 The author went through considerable effort trying to film Brownian motion under a microscope with limited success
- 🤯 Einstein published 3 groundbreaking papers in 1905 including one relating Brownian motion to atomic theory
- ❓ The equal number of molecules occupying the same volume of different gases at equal temperature and pressure is unintuitive
- 🧠 Brown, Einstein and Perrin uncovered profound truths by analyzing seemingly random processes
- 👩🏫 The author promotes an educational program from Jane Street aimed at high school graduates interested in math and computer science
Q & A
What produces the Brownian motion that Einstein studied?
-The random collisions of atoms and molecules with larger suspended particles causes the jittery Brownian motion.
Why did Robert Brown use quartz crystals in his early experiments on Brownian motion?
-To rule out the possibility that the motion he observed was due to anything biological or alive.
How did Einstein apply physics equations to Brownian motion?
-He assumed that the invisible molecules and visible particles followed the same physical principles, just differing in size. This allowed him to apply fluid physics equations.
What is Avogadro's number and why was it significant that Einstein's theory allowed its calculation?
-Avogadro's number specifies the number of atoms or molecules in a certain amount of a substance. Determining this allowed precise calculations of atomic and molecular masses.
What did Jean Perrin's experiments following Einstein's theory accomplish?
-Perrin experimentally confirmed Einstein's theory and calculated Avogadro's number as 7.15 x 10^23, very close to the modern value.
Why was 1905 such a significant year for Einstein?
-In 1905 alone Einstein published seminal papers on relativity, the photoelectric effect, and Brownian motion. This incredible outpouring of insights led to 1905 being called his annus mirabilis or 'miraculous year'.
What gases would contain the same number of atoms or molecules at a given temperature and pressure in equal volumes?
-All gases, regardless of the atomic or molecular weight, contain an equal number of atoms or molecules (Avogadro's number) when occupying the same volume under the same conditions.
What interested Einstein about the random walk character of Brownian motion?
-He realized that by observing particles over time and measuring how far they moved on average, he could calculate their diffusion coefficient.
How does the puzzle Jane Street created relate to the topics covered in the video?
-Just as Brownian motion seems chaotic but has an underlying order, the financial markets also can appear random but have deeper patterns that can be uncovered mathematically.
Why was Brown unsure at first that what he observed under the microscope was not something alive?
-He was originally viewing pollen particles which could perhaps move on their own. By also seeing motion in mineral crystals, dust, etc. he confirmed it was not biological.
Outlines

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowMindmap

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowKeywords

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowHighlights

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowTranscripts

This section is available to paid users only. Please upgrade to access this part.
Upgrade NowBrowse More Related Video

What Is Brownian Motion? | Properties of Matter | Chemistry | FuseSchool

Brownian Motion - Defintion, Example, Experiment, Applications

Brownian motion demonstration

SISTEM KOLOID (Part 2), Sifat-sifat Sistem Koloid
How do we know atoms are real? (Brownian Motion)

GCSE Chemistry - States of Matter & Changing State #21
5.0 / 5 (0 votes)